





Pancreatic exocrine insufficiency (PEI) is inadequate intraluminal pancreatic enzyme activity, due to insufficient enzyme production, insufficient enzyme activation, or early enzyme degradation leading to maldigestion and malabsorption of nutrients.4
PEI is commonly associated with

Abdominal pain

Flatulence

Weight loss

Bloating
Steatorrhea (fatty stools)
Pancreatic Enzyme Replacement Therapy (PERT) replaces the enzymes that pancreas make and help to treat Pancreatic exocrine insufficiency.

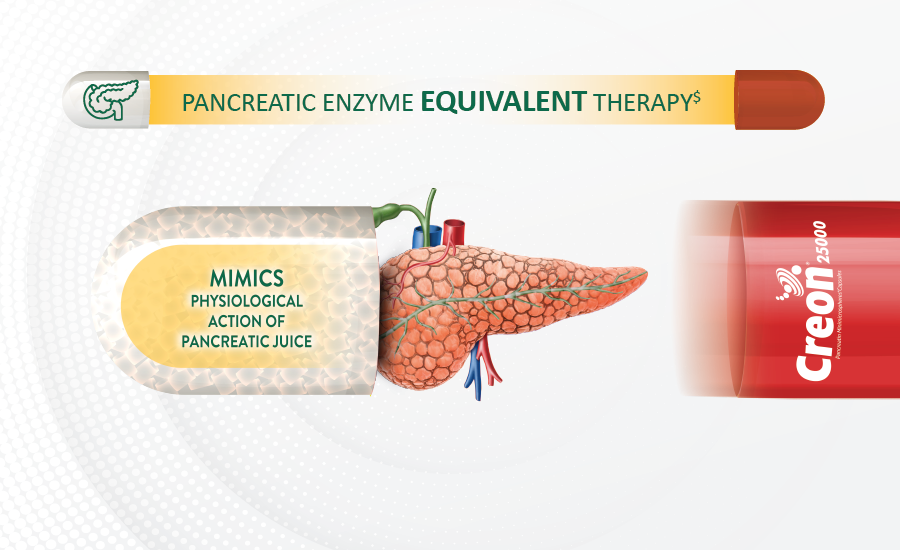
Creon contains lipase, amylase and protease enzymes and offers USFDA compliant lipase activity5,6
Adequate lipase, amylase, and protease5. Additional advantage of Phopholipase A2 and Cholesterolesterase
Gastric acid protection ensures smooth transition to duodenum without dilution in stomach5
Rapid Enzyme release at right pH for maximum lipase availability5
Phospholipase A2 |
|---|
Hydrolyzes |
Phospholipids |
Cholesterol Esterase |
|---|
Hydrolyzes |
Cholesteryl Esters |
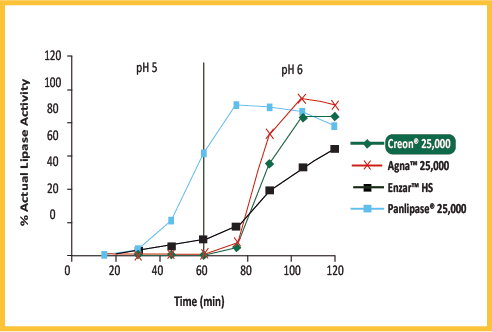
| BRAND | % LIPASE ACTIVITY |
|---|---|
| CREON® 25000 | 111% |
| AGNA 25000 | 77% |
| ENZAR HS | 93% |
| PANLIPASE 25000 | 55% |








(1) Shivaprasad, C., Pulikkal, A. A., & Kumar, K. M. (2015). Pancreatic exocrine insufficiency in type 1 and type 2 diabetics of Indian origin. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], 15(6), 616–619. https://doi.org/10.1016/j.pan.2015.09.018
(2) Hollemans, R. A., Hallensleben, N. D. L., Mager, D. J., Kelder, J. C., Besselink, M. G., Bruno, M. J., Verdonk, R. C., van Santvoort, H. C., & Dutch Pancreatitis Study Group (2018). Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], 18(3), 253–262. https://doi.org/10.1016/j.pan.2018.02.009
(3) Chaudhary A, Domínguez-Muñoz JE, Layer P, Lerch MM. Pancreatic Exocrine Insufficiency as a Complication of Gastrointestinal Surgery and the Impact of Pancreatic Enzyme Replacement Therapy. Dig Dis. 2020;38(1):53-68. doi: 10.1159/000501675. Epub 2019 Aug 16. PMID: 31422398; PMCID: PMC6979421.
(4) Shrikhande SV, Prasad VGM, Domínguez-Muñoz JE, Weigl KE, Sarda KD. In vitro Comparison of Pancreatic Enzyme Preparations Available in the Indian Market. Drug Des Devel Ther. 2021 Sep 7;15:3835-3843. doi: 10.2147/DDDT.S319949. PMID: 34522087; PMCID: PMC8434830.
(5) Shrikhande SV, et al. Drug Design, Development and Therapy. 2021;15:3835
(6) Kuhn RJ, et al. The Journal of Pediatric Pharmacology and Therapeutics. 2007;12(2):115-28.
(7) Internal Calculations based on Quintiles IMS database, Quintiles IMS Analytics Link MATO9 2016.
(8) Creon International Scientific Brochure
(9) SolvayPharmaceuticals. NDA 20-725 for Creon' (Pancrelipase Delayed-release Capsules) Briefing Document for December 2, 2008 Antiviral Drugs Advisory Committee. Available from-https://www fda gov/ohrms/dockets/ac/08/transcripts/2008-4402tI-Partl pdf. Accessed on 25 Oct.2017
(10) Creon Global sales aid GLCRE150051
(11) Mohan V, Poongothai S, Pitchumoni CS. Oral pancreatic enzyme therapy in the control of diabetes mellitus in tropical calculous pancreatitis. Int J Pancreatol. 1998 Aug;24(1):19-22. doi: 10.1007/BF02787526. PMID: 9746885
(12) Ramesh H, Reddy N, Bhatia S, Rajkumar JS, Bapaye A, Kini D, Kalla M, Thorat V. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. Pancreatology. 2013 Mar 1;13(2):133-9.
Copyright ©creon, All Right Reserved.