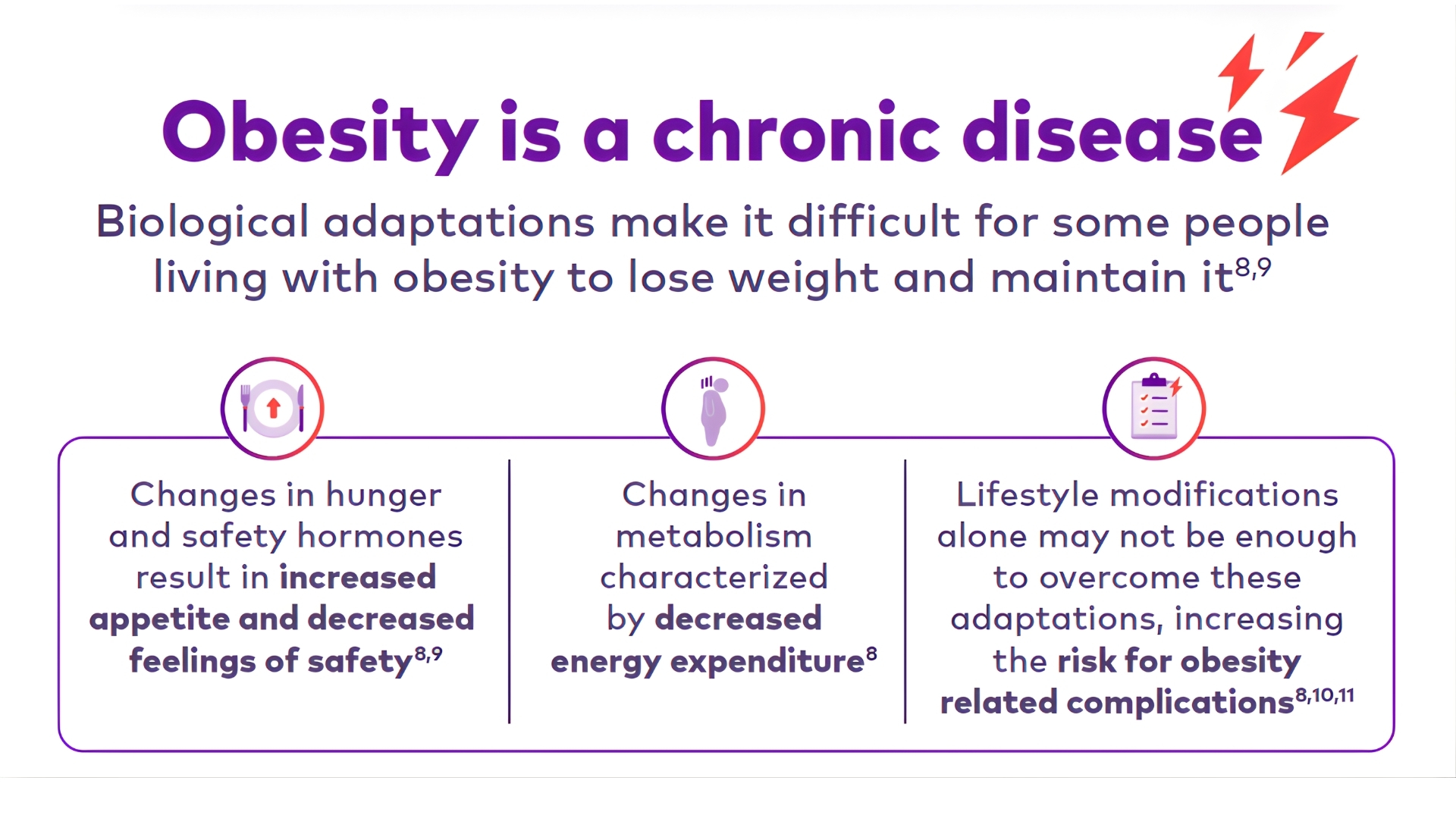
She has tried multiple times,
yet her attempts at losing weight and maintaining it have been unsuccessful

She is worried about her excess weight and risk of obesity-related complications*

She is starting to feel the physical and psychological consequences of excess weight in her daily life
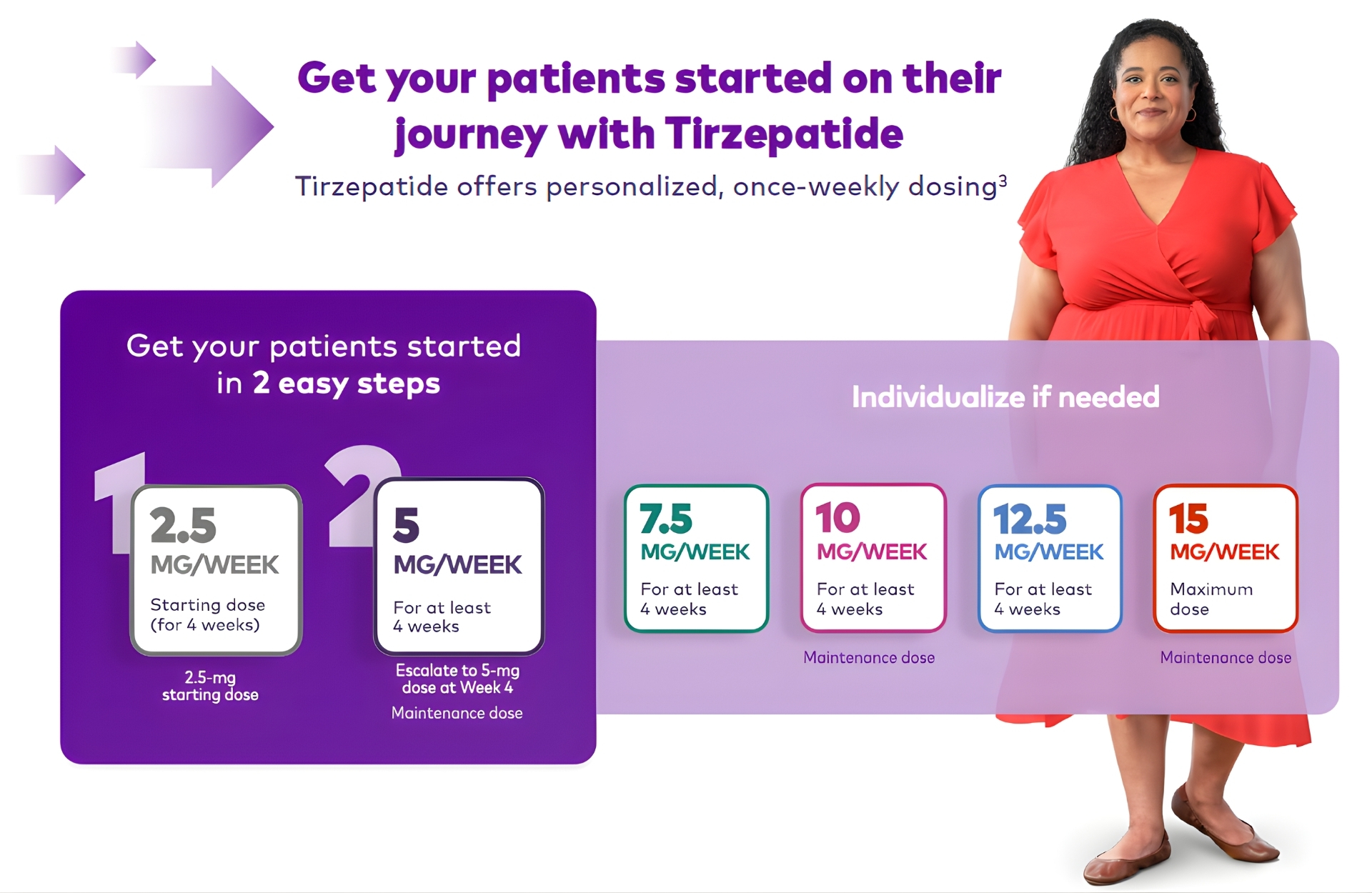
See how people like Anjali may achieve
their weight goals with Mounjaro®
Get started 
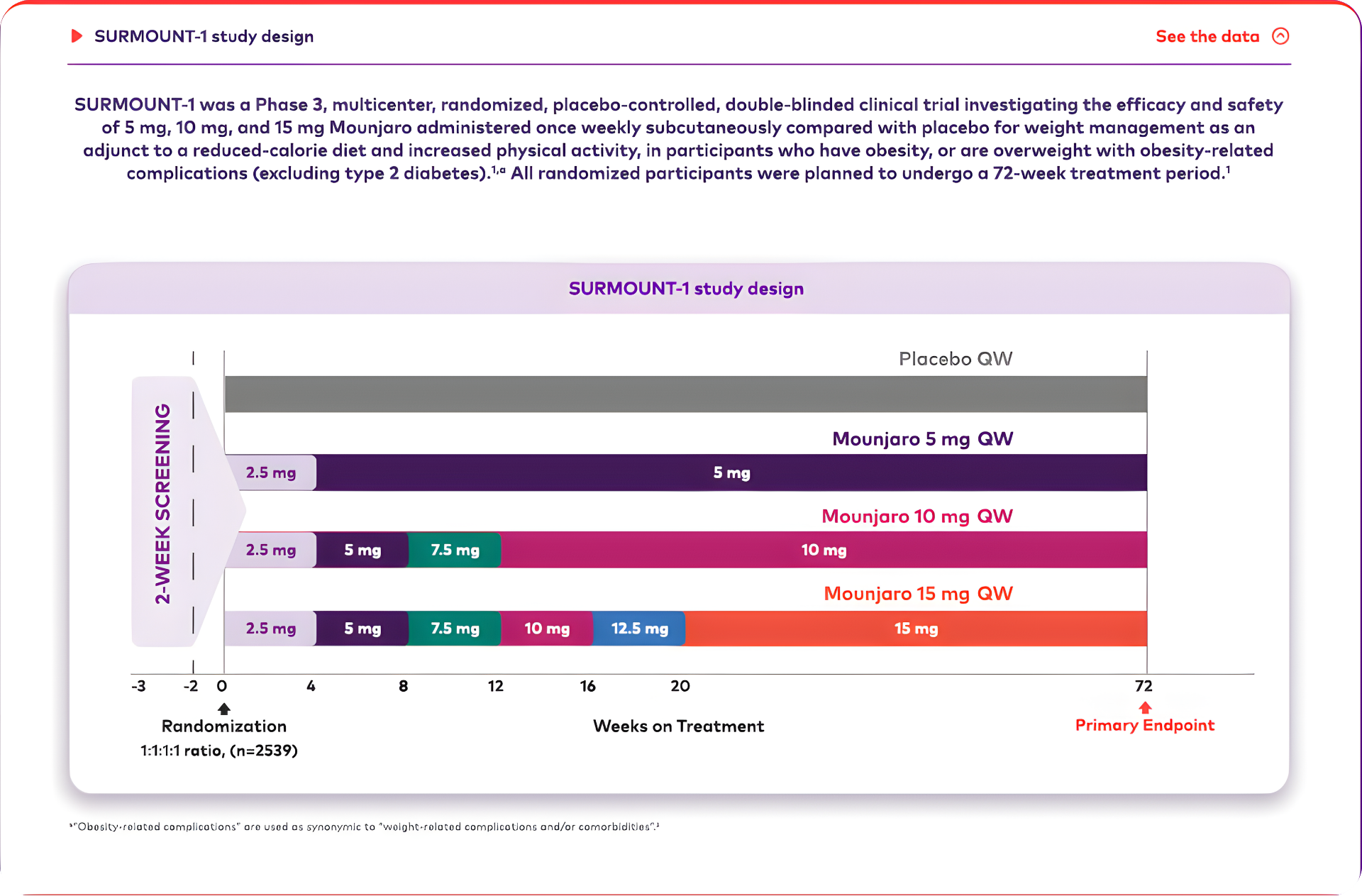
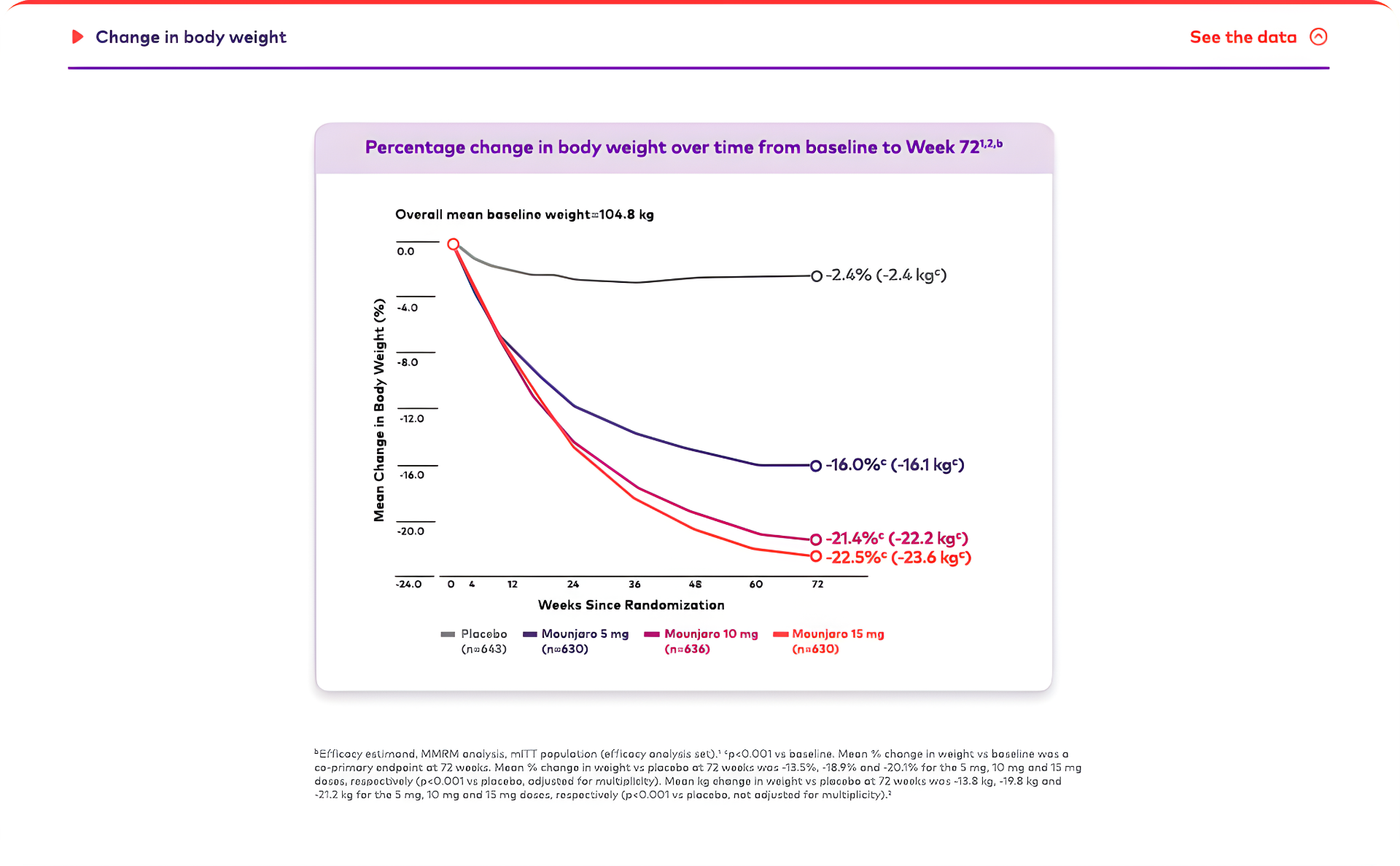
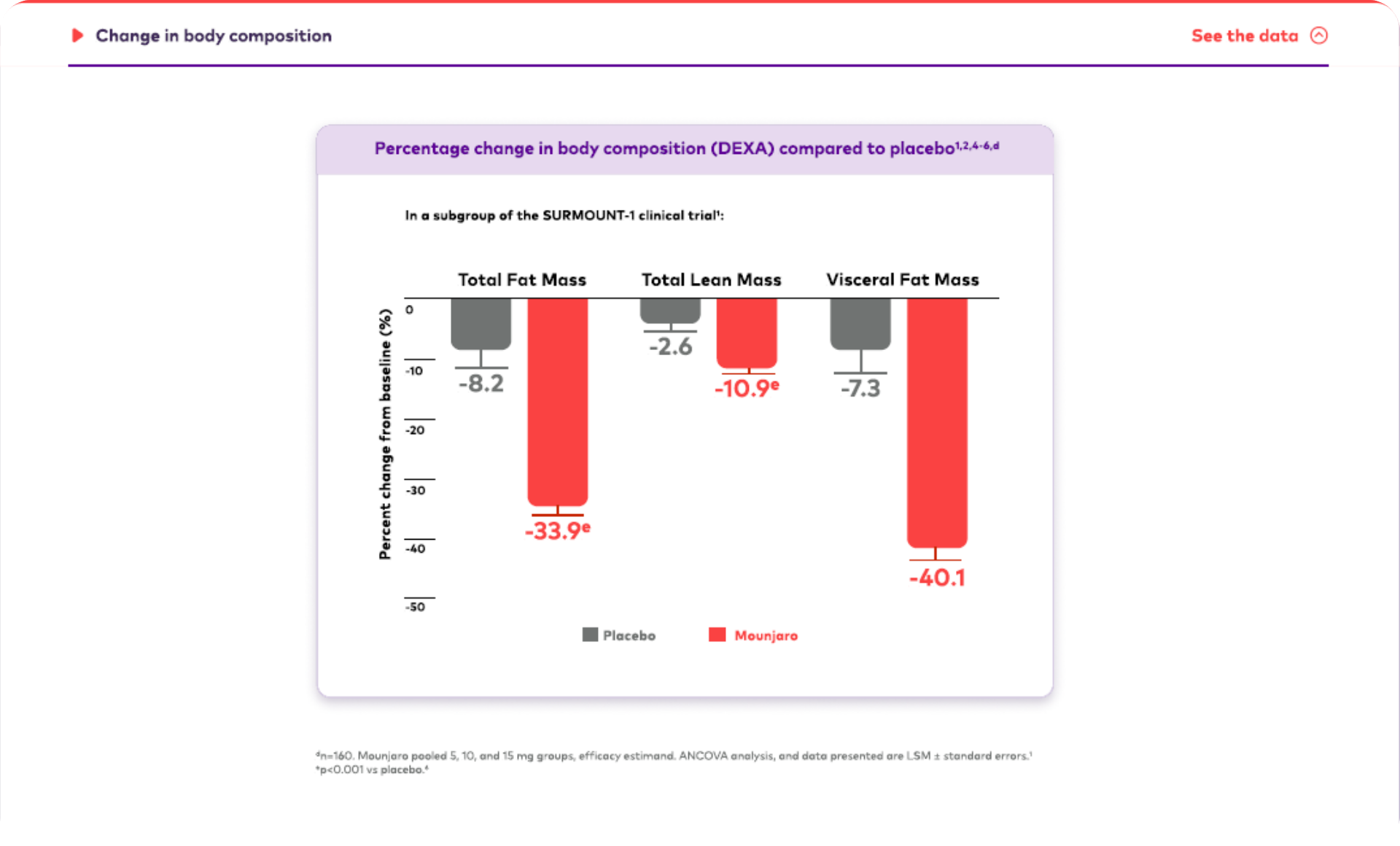
Mounjaro is the first and only GIP and GLP-1 receptor agonist approved for weight management that may help patients living with obesity achieve their weight goals.1-3,*
The role of GIP and GLP-1 receptors
- GIP and GLP-1 receptors are present in many body areas, including brain regions that regulate appetite1,a
- GIP receptors are present in adipose tissue and help regulate fat storage1,4
- GLP-1 regulates gastric emptying and feelings of satiety in the brain1,8
Brain
GIP activity8,b
Reduced food intake
GLP-1 activity6,8
Reduced food intake
Increased satiety
Subcutaneous
white adipose tissue
GIP activity4,8
Increased insulin sensitivity
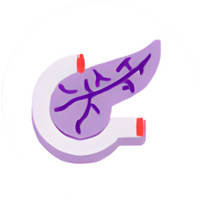
Pancreas
GIP activity9
Increased insulin secretion
Increased glucagon in a glucose-dependent way
GLP-1 activity9
Increased insulin secretion
Reduced glucagon secretion
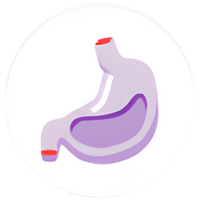
Stomach
GLP-1 activity7
Delayed gastric emptying

Mounjaro may have several effects on the body that help
support weight reduction in patients living with obesity1,10:
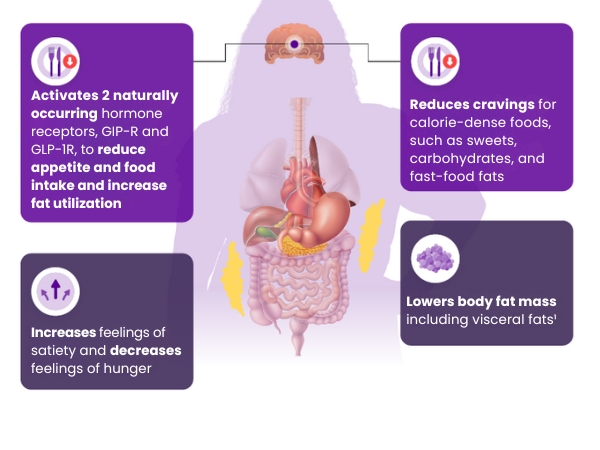


Mounjaro is the first and only GIP and GLP-1 receptor agonist approved for weight management that may help patients living with obesity achieve their weight goals.1-3,*
Type 2 diabetes mellitus
MOUNJARO® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:
• as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
• in addition to other medicinal products for the treatment of diabetes.
For study results with respect to combinations, effects on glycaemic control and the populations studied, see sections 4.4, 4.5 and 5.1.
Weight management
MOUNJARO® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of:
• ≥ 30 kg/m2 (obesity) or
• ≥ 27 kg/m2 to < 30 kg/m2 (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes, or type 2 diabetes mellitus).
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. The information provided in this section is intended solely for the use of registered medical practitioner. This material is being provided to healthcare professionals for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.
Mounjaro®, KwikPen® and Lilly are registered trademarks of Eli Lilly and Company. To be sold by retail under prescription of Endocrinologist or Internal Medicine Specialists only.
For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com
For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com.
For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001 India Ph.: +91-124-4753000/01 | www.lilly.com/in


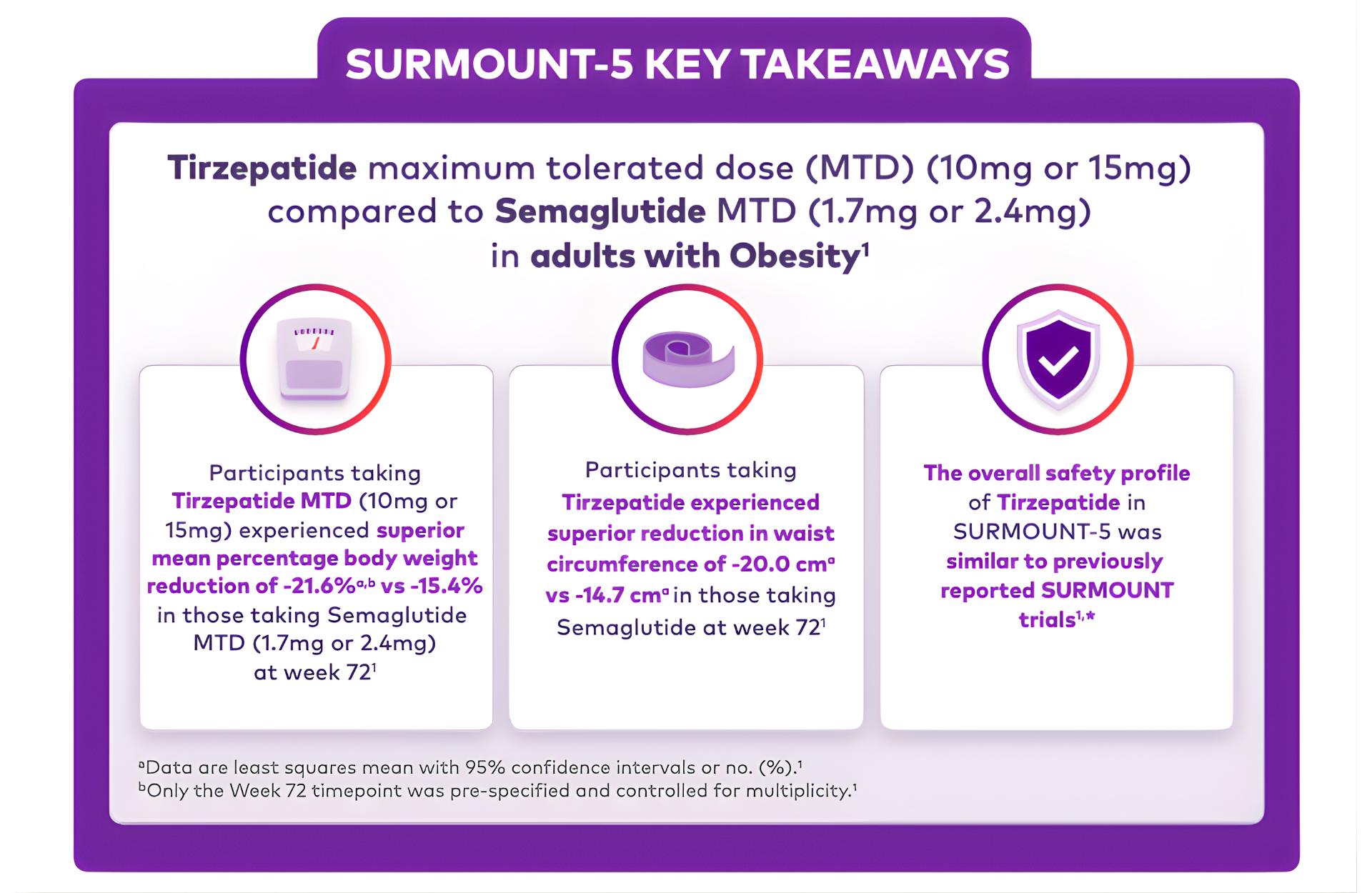
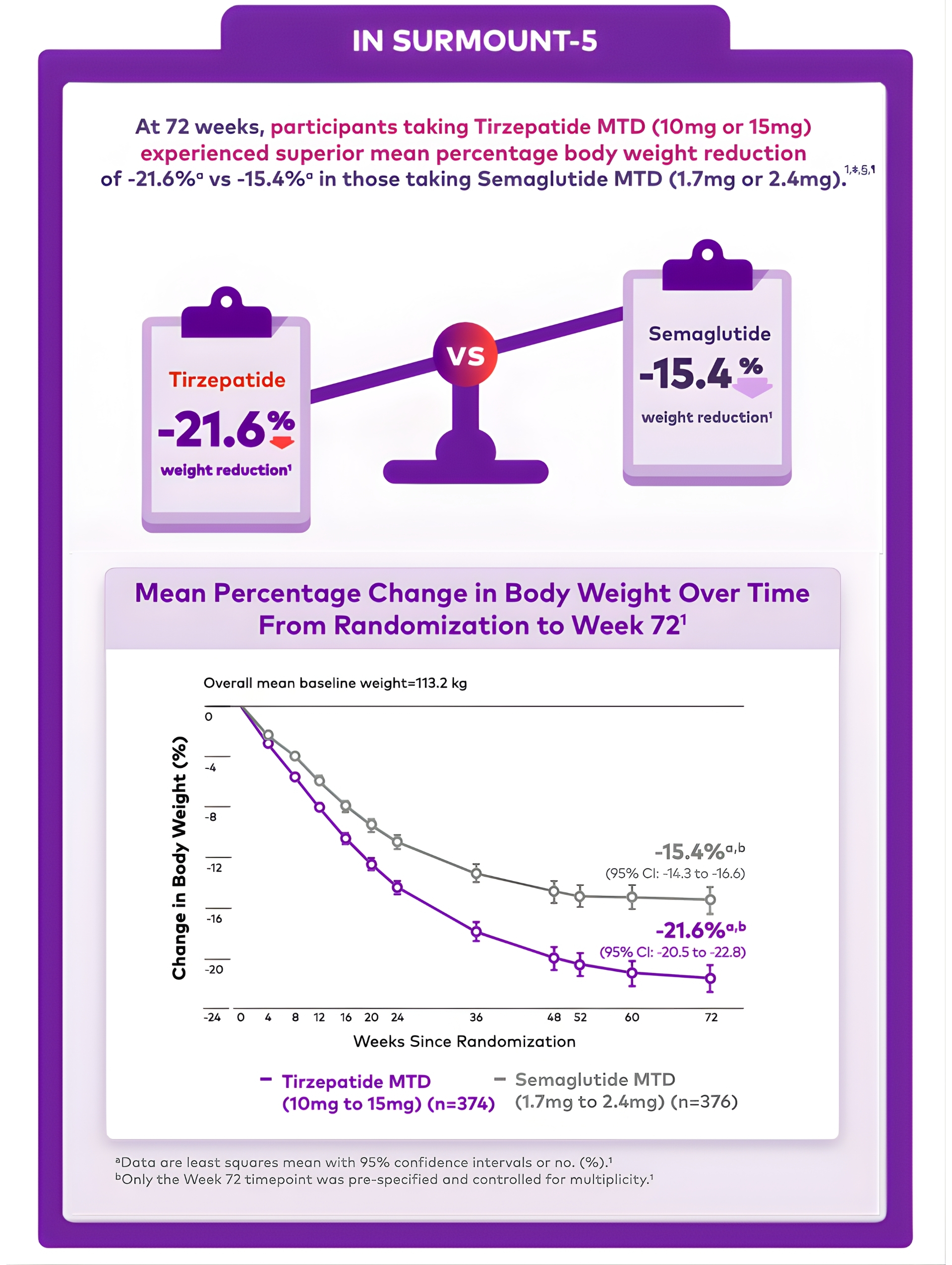
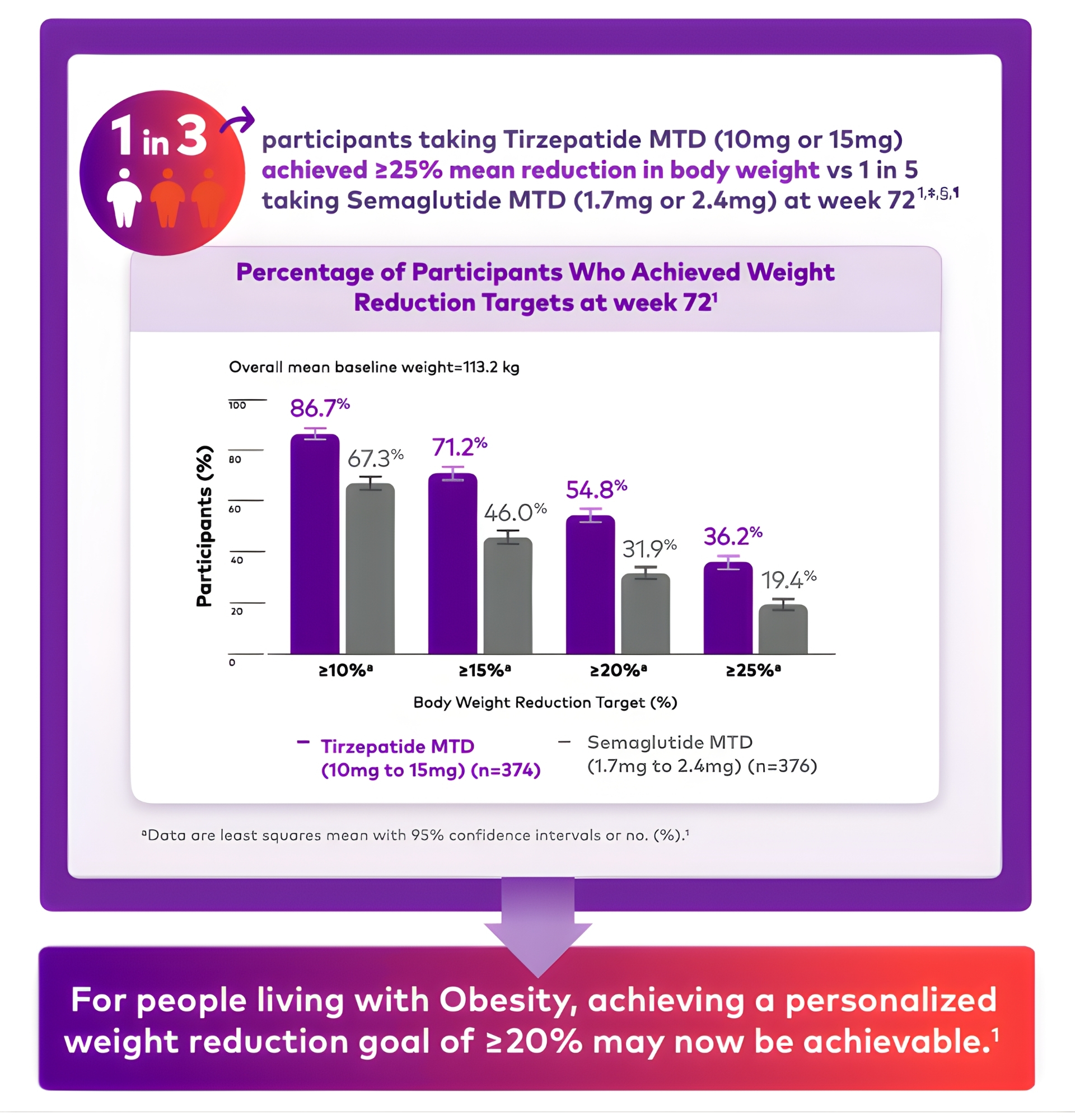
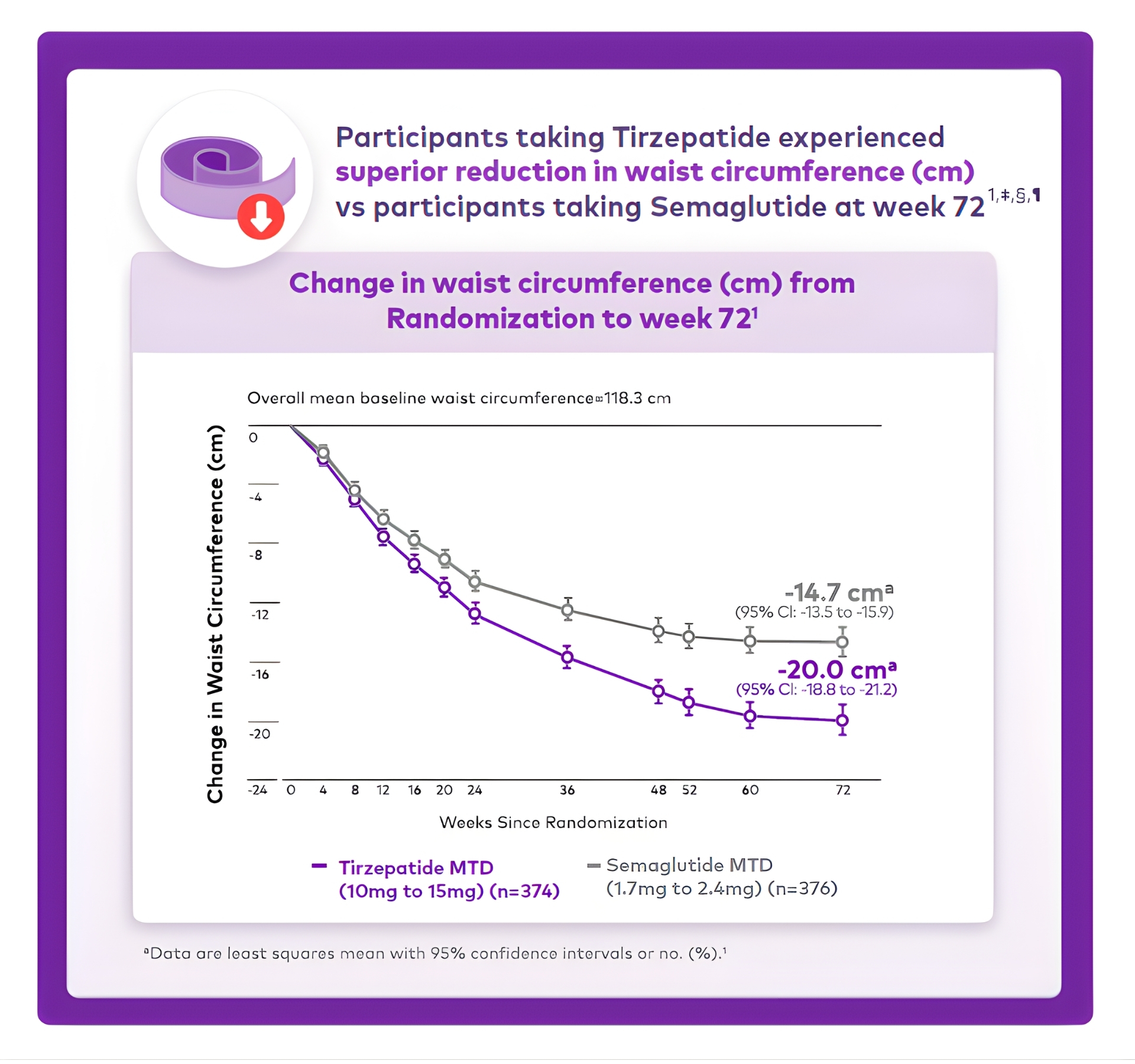
in both treatments groups1,#
(10mg to 15mg) (n=374)
(1.7mg to 2.4mg) (n=376)
Type 2 diabetes mellitus
MOUNJARO® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:
• as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
• in addition to other medicinal products for the treatment of diabetes.
For study results with respect to combinations, effects on glycaemic control and the populations studied, see sections 4.4, 4.5 and 5.1.
Weight management
MOUNJARO® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of
• ≥ 30 kg/m2 (obesity) or
• ≥ 27 kg/m2 to < 30 kg/m2 (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes, or type 2 diabetes mellitus).
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. The information provided in this section is intended solely for the use of registered medical practitioner. This material is being provided to healthcare professionals for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.
Mounjaro®, KwikPen® and Lilly are registered trademarks of Eli Lilly and Company. To be sold by retail under prescription of Endocrinologist or Internal Medicine Specialists only.
For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com
For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com.
For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001 India Ph.: +91-124-4753000/01 | www.lilly.com/in