
 Susten contains progesterone identical to that secreted by human ovary for Vaginal/Oral administration.
Susten contains progesterone identical to that secreted by human ovary for Vaginal/Oral administration. Progesterone is an essential hormone which plays an important role in woman’s normal reproductive cycle.
Progesterone is an essential hormone which plays an important role in woman’s normal reproductive cycle.  Essential during luteal phase to sustain pregnancy promotes shedding of endometrium due to its antiproliferative endometrial action and thereby helps in maintaining a regular menstrual cycle
Essential during luteal phase to sustain pregnancy promotes shedding of endometrium due to its antiproliferative endometrial action and thereby helps in maintaining a regular menstrual cycle 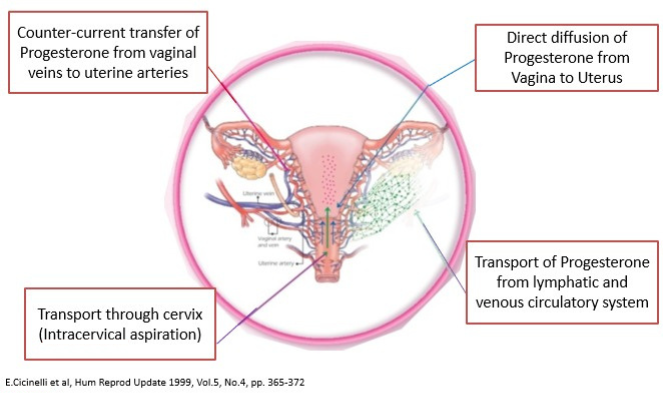
Vaginal route leads to ~ 10 fold higher progesterone delivery compared to I.M. injections, avoids first pass hepatic metabolism, associated with minimal systemic side effects2.








Clinical Evidence in Luteal Phase Support:
Key Highlights:
A systematic review and meta-analysis based on randomized controlled trials analysed the effect of progesterone supplementation for LPS on the reproductive outcomes of patients undergoing natural cycle frozen embryo transfer (NC-FET). Vaginal progesterone supplementation was associated with increased LBR (RR, 1.42; 95% CI, 1.15-1.75) and CPR (RR, 1.30, 95% CI, 1.07-1.57) in patients undergoing NC-FET cycles. Subgroup analysis showed that vaginal progesterone supplementation was associated with higher LBR and CPR in tNC-FET cycles (true NC-FET cycles). 15
Key Highlights:
A 2022 study analyzed the impact on live birth rates (LBRs) of the individualized luteal phase support (termed iLPS) in patients with low serum progesterone (P) levels compared with patients without iLPS.
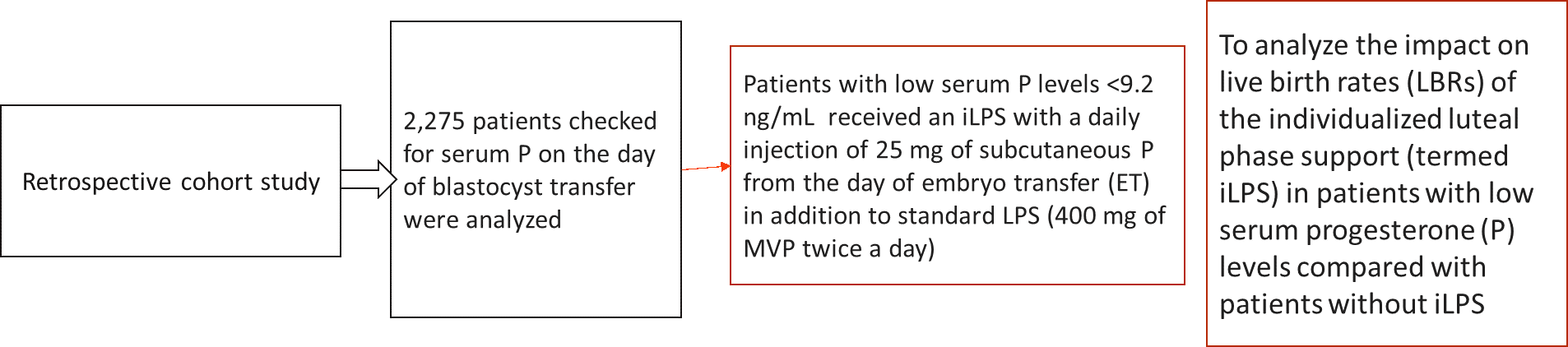
Results were as follows:
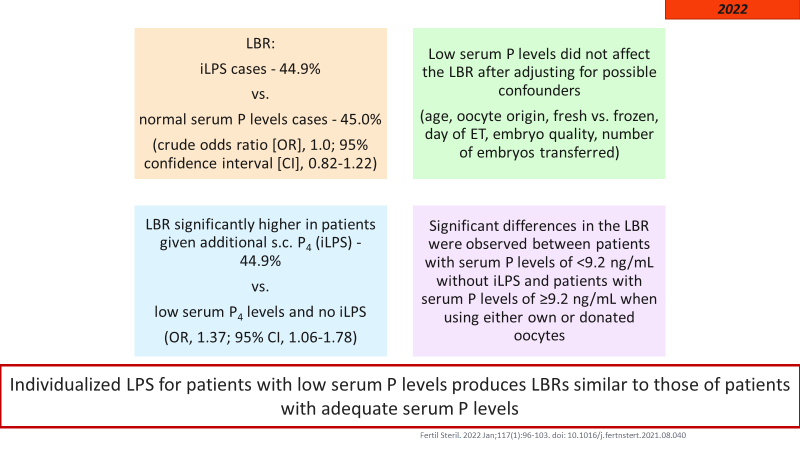
The authors concluded that Individualized LPS (with vaginal micronized progesterone and subcutaneous (s.c.) progesterone) for patients with low serum P levels produces LBRs similar to those of patients with adequate serum P levels
Key Highlights:
A Delphi consensus was conducted to evaluate global expert opinions on key aspects of assisted reproductive technology (ART) treatment, wherein it was established that Vaginal progesterone therapy represents the gold standard for luteal-phase support. 16
Key Highlights:
A 2014 study evaluated endometrial morphology on day 21 progesterone administration in women with primary ovarian failure and correlated serum levels with histological findings17. The results were as shown below
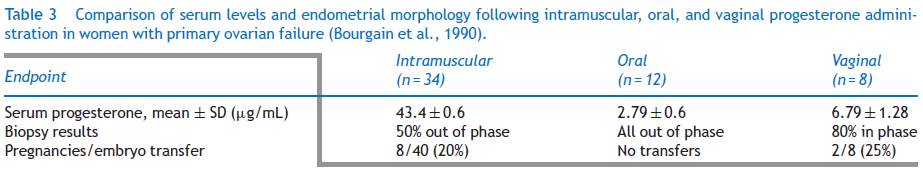
Therefore, it was concluded that Endometrial response most closely matched a natural cycle after vaginal progesterone administration.
Furthermore, Vaginal Progesterone was found to be effective for priming endometrial receptivity in DE-ART and assuring LPS in ART whereas the synthetic progestin dydrogesterone, was ineffective for priming a predecidualized endometrium in mock DE-ART cycles.18
With regards to placental circulation, vaginal progesterone administration, but not oral dydrogesterone treatment, resulted in decreased in spiral artery pulsatility & resistance index & systolic/diastolic ratio.19
ACOG Practice Bulletin No. 234 (April 2023), “Prediction and Prevention of Spontaneous Preterm Birth” recommends “Vaginal progesterone may be considered as a treatment option for patients with a history of preterm birth, singleton gestation, and a shortened cervix.” 20
FIGO Working Group recommended offering either daily vaginal progesterone in preventing preterm births.21
NICE guidelines22 recommend to offer a choice of prophylactic vaginal progesterone as an option to women who have both:
When using vaginal progesterone, start treatment between 16+0 and 24+0 weeks of pregnancy and continue until at least 34 weeks.
SFMF guidelines23 recommend “In women with singleton gestations, no prior PTB, and short CL ≤20mm at ≤24 weeks, vaginal progesterone, either 90-mg gel or 200-mg suppository, is associated with reduction in PTB and perinatal morbidity and mortality, and can be offered in these cases” and “Practitioners who choose to screen low-risk singleton gestations may consider offering vaginal progesterone, either 90-mg gel or 200-mg suppositories, for short TVU CL ≤20mm at ≤ 24 weeks.”
EAPM guidelines recommend that Asymptomatic women with a sonographically short cervix ( 25 mm) at mid gestation, either with singleton or twin pregnancy and regardless of their obstetrical history should be offered vaginal progesterone treatment for the prevention of preterm birth and neonatal morbidity.24
FOGSI recommends vaginal progesterone in a dose of either 100 to 200 mg micronized progesterone capsules vaginally nightly. 25
1) J Clin Diagn Res. 2016 Feb; 10(2): QE01–QE04. , 2) Bulletti C, de Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, Franceschetti F. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997 May;12(5):1073-9. doi: 10.1093/humrep/12.5.1073. PMID: 9194669., 3) Susten Capsules prescribing information., 4) https://www.fogsi.org/wp-content/uploads/2015/11/Progesterone-position-paper-Oct-2015.pdf, 5) https://www.fogsi.org/wp-content/uploads/fogsi-focus/Progesterone-book.pdf accessed on 12th June 2023, 6) Malik S, et al. J Chin Diagn Res. 2016 Feb; 10(2);QE01-4, 7) Gian Carlo Di Renzo et al.; Best Pract Res Clin Obstet Gynaecol. 2020 Nov;69:2-12, 8) As per Prescribing information , 9) *Data on file, 10) Press Release January 22 2016,Information : Approval has been granted for UTROGESTAN Vaginal Capsules 200mg , 11) Australian Public Assessment Report for Progesterone June 2017 (AusPAR Prometrium/Utrogestan Besins Healthcare Australia Pty Ltd. PM -2014-03908-1-5 Final 1 June 2017), 12) https://www.tga.gov.au/sites/default/les/ausparprogesterone-170601.pdf accessed on 9th Feb 2023 , 13) Number of Studies/Clinical Papers Reecting Progesterone on Pubmed accessed on 9th Feb 2023, 14) Katarzyna J Siemienowicz et al., Sci Rep. 2020 Dec 14;10(1):21920., 15) Fertil Steril. 2023 Apr;119(4):597-605. doi: 10.1016/j.fertnstert.2022.12.035. Epub 2022 Dec 24. PMID: 36574915. , 16) Front Endocrinol (Lausanne). 2021; 12: 675670 , 17) Reprod Biomed Online. 2014 Dec;29 Suppl 1:S1-14; quiz S15-6 , 18) Fertil Steril 2013;100:860–6 , 19) Czajkowski K, et al. Fertil Steril. 2007 Mar;87(3):613-8, 20) https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/04/updated-guidance-use-of-progesterone-supplementation-for-prevention-of-recurrent-preterm-birth accessed on 12th June 2023 , 21) Jacobsson B, Simpson JL; FIGO Working Group for Preterm Birth. FIGO good practice recommendations for reducing preterm birth and improving child outcomes. Int J Gynaecol Obstet. 2021 Oct;155(1):1-4. doi: 10.1002/ijgo.13863. PMID: 34520060, 22) https://www.nice.org.uk/guidance/ng25/resources/preterm-labour-and-birth-pdf-1837333576645 accessed on 14th June 2023 , 23) https://www.smfm.org/publications/87-progesterone-and-preterm-birth-prevention-translating-clinical-trials-data-into-clinical-practice accessed on 14th June 2023 , 24) Di Renzo GC, Cabero Roura L, Facchinetti F, Helmer H, Hubinont C, Jacobsson B, Jørgensen JS, Lamont RF, Mikhailov A, Papantoniou N, Radzinsky V, Shennan A, Ville Y, Wielgos M, Visser GHA. Preterm Labor and Birth Management: Recommendations from the European Association of Perinatal Medicine. J Matern Fetal Neonatal Med. 2017 Sep;30(17):2011-2030. doi: 10.1080/14767058.2017.1323860. PMID: 28482713 , 25) https://www.fogsi.org/wp-content/uploads/fogsi-focus/Progesterone-book.pdf accessed on 12th June 2023.