Overview
About Cadomer
Cadomer (Cadexomer Iodine) is yellow-brown colored ointment containing cadexomer iodine (50% w/w) equivalent to 0.9% w/w available iodine.
Cadexomer Iodine is used in external wounds including leg ulcers, pressure ulcers and diabetic ulcers, infected traumatic and surgical wounds. This medicine effectively treats infection by controlling the growth of bacteria, fungi and viruses. It comes in ointment form and should be used for external use only
Cadomer Contains:-
Cadexomer
- Absorbs exudate not less than 2.5 ml/gm
- Disrupts & prevents biofilm reformation
Iodine
- Broad Spectrum antimicrobial
- Sustain release action up to 72 hrs
Poloxmer
- Surface active agents that disrupts biofilm
- Soften & removes cellular debris from wound bed
Indications of Cadomer
Cadomer is indicated for the treatment of exuding wounds such as leg ulcers, pressure ulcers and diabetic ulcers, infected traumatic and surgical wounds.
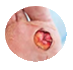
Diabetic Foot Ulcer
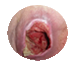
Pressure or Decubitus Ulcer
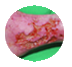
Venous Stasis Ulcer
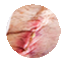
Infected Traumatic and Surgical Wounds
Evolution of Iodine In Wound Care
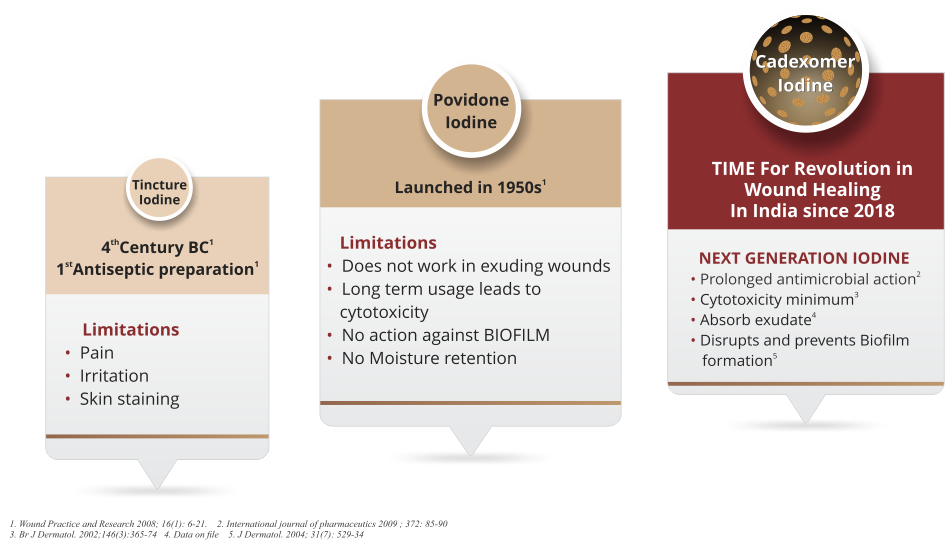
How Cadexomer Iodine Is Superior To Povidone Iodine?
| Parameters | Cadexomer Iodine | Povidone Iodine |
|---|---|---|
| Biofilm Disruption | Yes | No |
| Biofilm Prevention | Yes | No |
| Dressing Frequency | Once in 3 Days | Daily |
| Exudate Absorption | Yes | No |
| Efficacy in Exuding Wounds | High | Low |
| Iodine Release | Sustained | Immediate |
| Cytotoxicity | Low | High |
| Pharmacological Debridement | Yes | No |
| Antiseptic Action | Yes | Yes |
| Cost of Dressing Therapy | Low | High |
2. Acta Derm Venereol (stockh) 1996; 76; 231-235
Time Model Is Vital For Wound Healing
Cadexomer Iodine Addresses Time Model

TISSUE DEBRIDEMENT
Autolytic debridement helps disrupt biofilm

INFECTION CONTROL
Prolonged antimicrobial action up to 72 hr.

Moisture Balance
Improves granulation tissue formation

EDGE Margin Response
Early wound closure
Dose and Method of Administration
DOSAGE AND DIRECTIONS FOR USE
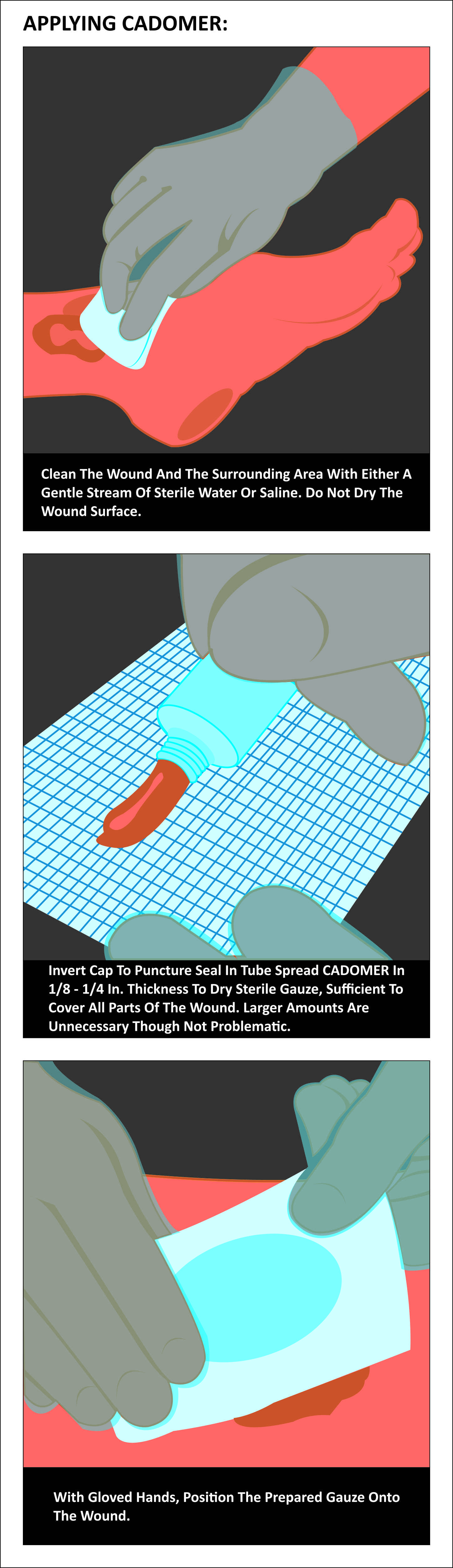
- Clean the wound with sterile water or saline. Do not dry the wound surface.
- Apply Cadomer ointment to dry sterile gauze, ensuring an adequate amount to cover the entire wound to a depth of 3 mm.
- Place secondary dressing over the wound with a gloved finger, smoothen over the dressing and spread the ointment underneath to the shape of the ulcer and to a minimum depth of 3 mm.
- A single application of Cadomer should not exceed 50 g. The total amount of Cadomer used in one week should not exceed 150 g.
- The duration of treatment should not exceed 3 months in any single course of treatment.
REMOVAL OF DRESSING
- Remove secondary dressing.
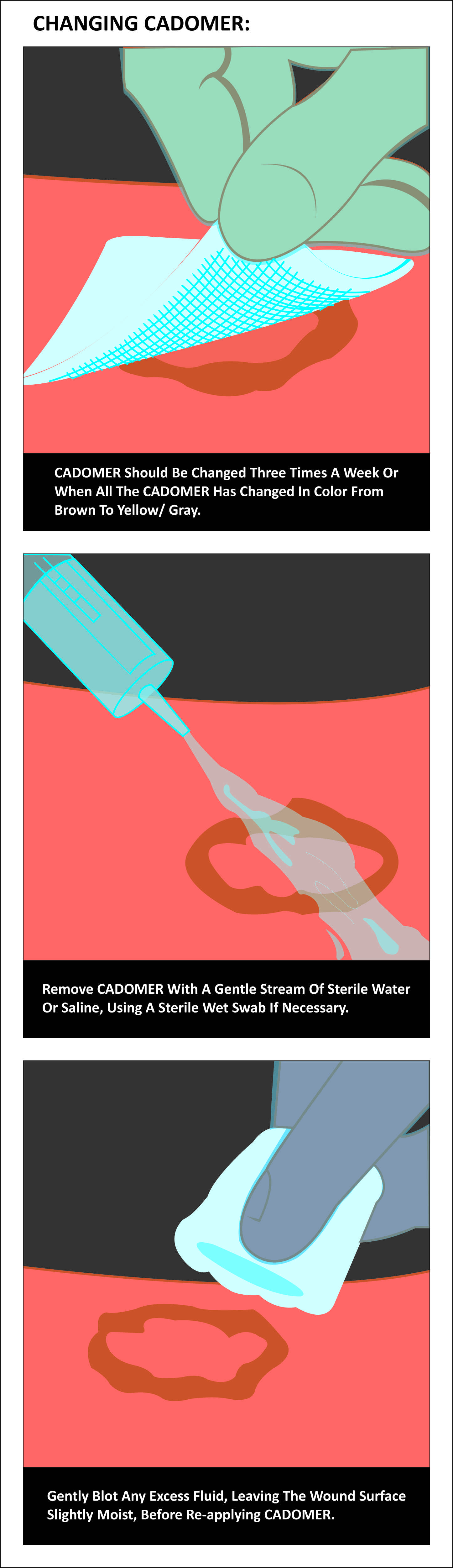
- Loss of dark-brown coloration confirms need to change dressing (usually 2-3 times a week or daily if wound is discharging heavily).
- If necessary, soak dressing for a few minutes with sterile water or saline, then remove.
- Remove Cadomer with a gentle stream of sterile water or saline, using a sterile wet swab if necessary.
- Gently blot excess fluid, leaving wound surface slightly moist, before reapplying Cadomer.
- The number of applications should be reduced as the exudate diminishes.
Contraindications
- Patients with known or suspected iodine sensitivity.
- Hashimoto's thyroiditis.
- Patients with history of Graves' disease or non-toxic nodular goitre.
- Pregnant or lactating womem.
Warnings and Precautions
Iodine may be absorbed systematically, when large wounds are treated. Patients with severely impaired renal function or a past history of any thyroid disorder are more susceptible to alterations in thyroid metabolism with chronic Cadomer ointment therapy. In endemic goitre there have been isolated reports of hyperthyroidism associated with exogenous iodine. It has been observed occasionally that an adherent crust can form when Cadomer ointment is not changed at required frequency. Cadomer is not effective in treating dry wounds.
Undesirable Side Effects/Overdose
Patients treated with Cadexomer Iodine Ointment may experience a transient smarting or pain within the first hour after application. Minor reddening or swelling around the wound may occur without necessarily being an allergic reaction. Contact allergy and local edema have been reported. There have been no reported over dosages. In case of excessive topical use of Cadomer the treatment should be stopped, the area is washed and symptomatic treatment is initiated.
Download the Product Leaflet, to see the detailed information on the Undesirable Side Effects/Overdose.
Pharmacodynamic and Pharmacokinetic Properties
Download the Product Leaflet, to see the details on Pharmacodynamic and Pharmacokinetic Properties of Cadomer.