Nebulized Colistin is effective in VAP
Meta-analysis shows aerosolized colistin is associated with improved outcomes (significant improvement in clinical response, microbiological eradication and infection-related mortality) in the treatment of VAP.4
Colistin when given through the nebulized route offers to target-specific drug delivery, superior action and lesser side effects.5
Colistin has high susceptibility against MDR and XDR pathogens.6
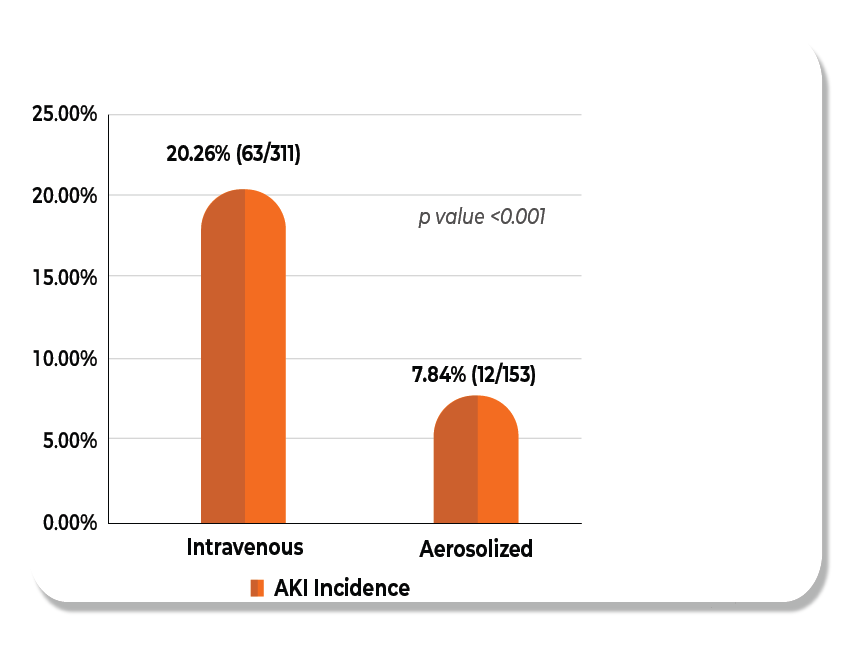
The addition of Nebulized colistin ensure minimal or no risk of Nephrotoxicity.7
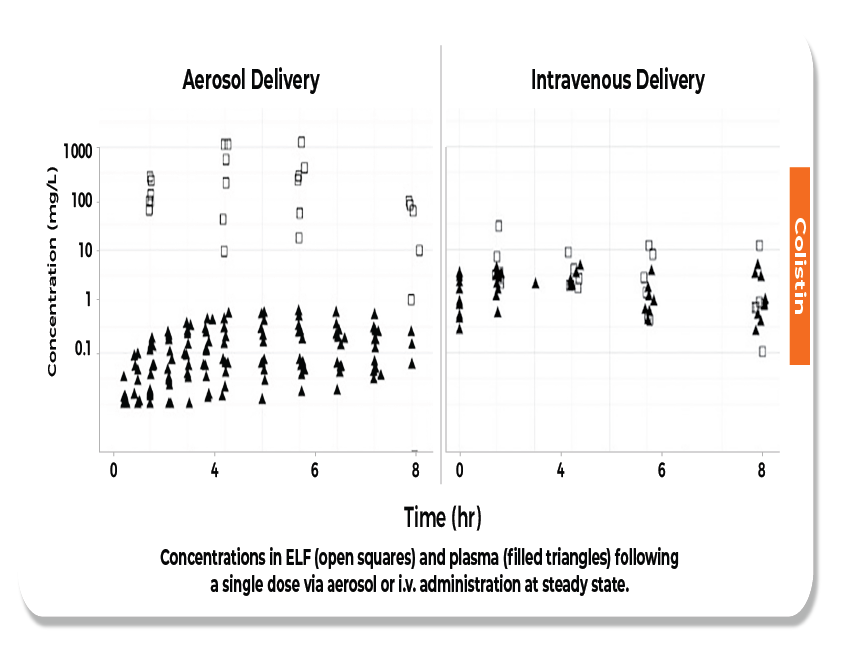
Nebulized Colistin achieves higher ELF and lowers plasma concentration after aerosol delivery.8
Nebulized Colistin has 10 times higher sputum concentration than the MIC breakpoint for P.aeruginosa.9
Colistin in VAP - Why Aerosol is better than IV?
than IV delivery18
What is VAP?
VAP usually occurs 48–72 hrs after mechanical ventilation and is related to the increased incidence of multidrug-resistant (MDR) infections, prolonged mechanical ventilation, increased antibiotic usage, and patient stay in the hospital.11
| Clinical Diagnostic Strategies of VAP | ||
|---|---|---|
| If there is underlying pulmonary or cardiac disease, two serial x-rays demonstrating at least one of the following: A) New progressive infiltrate B) Consolidation C) Cavitation If there is no underlying pulmonary or cardiac disease, one definitive imaging test result is acceptable | Blood count of white blood cell not more than 4 or at least 12 x 103 cells/mm3 | A) Temperature < 36°Cor > 38°C B) New onset or increase of purulent aspirates C) Wheezing, rales, or rhonchi D) Apnea, tachypnea, nasal flaring with retractions of the chest wall or nasal flaring with grunting E) Worsening gas exchange ( oxygen desaturation, or increases oxygen requirement, or increased ventilator demand) |
| Type of pathogens | |
|---|---|
| Bacterium | Methicillin-sensitive staphylococcus aureus MRSA Pseudomonas aeruginosa Acinetobacter baumannii streptococcus pneumoniae Escherichia coli klebsiella pneumoniae Anaerobic bacteria legionella ESBL-PE |
| Fungus | Aspergillus candida |
| Virus | Influenza respiratory syncytial virus |
Prevention
- Minimizing exposure to mechanical ventilation. 13
- Personal oral hygiene care helps reduce risk of VAP. 14
- Subglottic suctioning reduces VAP by 45%.15
- Keeping the head of the bed elevated between 30 and 45 degrees.16
- Probiotic bacteria can decrease development of VAP.17
*IDSA Guidelines recommend inhalational colistin for management of VAP
What is Colistin?
Colistin belongs to the polymyxin group of antibiotics and is also known as polymyxin E. It has good susceptibility against resistant gram-negative pathogens. Hence, this becomes a drug of choice, especially in this MDR / XDR (extensively drug-resistant) era or when carbapenem-resistant pathogens are suspected.3 Inhaled colistin overcomes the limitations of systemic antibiotics by less systemic penetration and may result in a lower incidence of nephrotoxicity. Therefore, Nebulised Colistin- a better option against resistant pathogens.
Reduces sputum colonization
Reduces pulmonary inflammation

Reduces pulmonary exacebation

Improves quality of life
Indications of Colistin
Cystic fibrosis
Cystic fibrosis is caused by a gene mutation leading to dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It affects multiple organ systems—the lungs, pancreas, upper airways, liver, intestine, and reproductive organs—to varying degrees.20
Bronchiectasis
Bronchiectasis is a chronic, debilitating respiratory condition that affects people of all ages. Patients have daily excessive sputum and associated symptoms, recurrent chest infections and impaired health-related quality of life.21
MDR Lung Infection
Colistin still maintains its susceptibility; so, this becomes a drug of choice, especially in this MDR/XDR (extensively drug-resistant) era or when carbapenem-resistant pathogens are suspected.22
Colistin overview
Colistin has good susceptibility against resistant gram- negative pathogens and has become a drug of choice in MDR /XDR era or when carbapenem-resistant pathogens are suspected.
Colistin belongs to the polymyxin group of antibiotics, and is also known as polymyxin E. It has good susceptibility against resistant gram-negative pathogens. According to the Center for Disease Dynamics, Economics & Policy (CDDEP) 2017 data, resistance rates of carbapenems against Escherichia coli, P. aeruginosa, Klebsiella and Acinetobacter baumannii are 18%, 30%, 59% and 77%, respectively.
The Indian Council of Medical Research (ICMR) initiated the Antimicrobial Resistance Surveillance & Research Network (AMRSN) to enable compilation of data from India on six pathogenic groups that show antimicrobial resistance. Six nodal centres for each pathogenic group were identified in four tertiary care hospitals. According to this ICMR 2018 data, there is a predominance of resistant gram-negative pathogens that are responsible for 90% of respiratory infections. Most of the routinely prescribed antibiotics are However, colistin still maintains its susceptibility; so, this becomes a drug of choice, especially in this MDR/XDR (extensively drug-resistant) era or when carbapenem-resistant pathogens are suspected. Inhaled colistin overcomes the limitations of systemic antibiotics by less systemic penetration and may result in lower incidence of nephrotoxicity. In this view, nebulised colistin can be considered as a better option against resistant pathogens. resistant against these pathogens. The following table showed the resistance rate of gram-negative bacilli against routinely prescribed antibiotics.
Indications
Colistin is used in the treatment of Cystic Fibrosis and Bronchiectasis.
For treatment by inhalation in P. aeruginosa lung infection in patients with Cystic Fibrosis.
Use in Special Population
Patients with Renal Impairment
Use with caution in renal impairment as colistimethate sodium is renally excreted.
Patients with Hepatic Impairment
No data available.
Pregnant Women
There are no adequate data on the use of colistimethate sodium in pregnant women. Single-dose studies in human pregnancy show that colistimethate sodium crosses the placental barrier and, hence, should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Lactating Women
Colistimethate sodium is secreted in breast milk. Colistimethate sodium should be administered to breastfeeding women only when clearly needed.
Geriatric Patients
Elderly patients are more likely to have decreased renal function; hence, care should be taken in dose selection and it may be useful to monitor renal function.
Dose and Method of Administration
Powder for solution for injection, infusion and inhalation 10,00,000 IU/20,00,000 IU. The following recommended doses are for guidance only and should be adjusted according to clinical response:
- Children (>2 years of age)
500,000 to 1 million units twice daily. - Children (>2 years of age) and Adults.
1–2 million units twice daily.
Colistin powder is dissolved in 2–4mL of sterile Water for Injections or 0.9% Sodium Chloride IV infusion for use in a nebuliser attached to an air/oxygen supply.
Contraindications
Colistin powder is contraindicated in patients with a known hypersensitivity to colistimethate sodium (colistin) or polymyxin B and patients with myasthenia gravis.
Warnings and Precautions
- Colistin powder is not to be used in food-producing animals, poultry, aqua farming and animal feed supplements.
- Use with extreme caution in patients with porphyria.
- Use with caution in renal impairment as colistimethate sodium is renally excreted.
Colistin is not to be used in food-producing animals, poultry, aqua farming and animal feed supplements. Use with extreme caution in patients with porphyria. Nephrotoxicity or neurotoxicity may occur if the serum concentration is exceeded. Use with caution in renal impairment as colistimethate sodium is renally excreted. It is advisable to assess baseline renal function and to monitor during treatment. Bronchospasm if occurred may be prevented or treated with the appropriate use of beta2-agonists. If troublesome, treatment should be withdrawn.
Drug interactions
- Concomitant use of colistimethate sodium with aminoglycoside antibiotics such as gentamicin, amikacin, netilmicin and tobramycin having neurotoxic and/or nephrotoxic potential should be avoided.
- There may be an increased risk of nephrotoxicity if given concomitantly with cephalosporin antibiotics.
- Neuromuscular-blocking drugs and ether should be used with extreme caution in patients receiving Neuromuscular-blocking drugs and ether should be used with extreme caution in patients receiving colistimethate sodium.
Undesirable Effects
After systemic administration, nephrotoxicity and neurotoxicity is a major concern. Incidence of nephrotoxicity maybe lower when administered by the inhalation route due to less systemic absorption. Inhalation may induce coughing or bronchospasm. Sore throat or mouth has been reported and may be due to Candida albicans infection or hypersensitivity. Skin rash may also indicate hypersensitivity; if this occurs, treatment should be withdrawn.
Adverse Reactions
- Nephrotoxicity and Neurotoxicity can occur after systemic administration Inhalation may induce coughing or bronchospasm.
- Sore throat or mouth has been reported and may be due to Candida albicans infection or hypersensitivity.
- Skin rash may also indicate hypersensitivity; if this occurs, treatment should be withdrawn.
Overdose
Overdose can result in neuromuscular blockade that can lead to muscular weakness, apnoea, and possible respiratory arrest. Overdose can also cause acute renal failure characterised by decreased urine output and increased serum concentrations of blood urea nitrogen (BUN) and creatinine.
Pharmacodynamic and pharmacokinetic properties
- It has good activity against P. aeruginosa and its spectrum of activity also comprises E. coli, Enterobacter spp., Salmonella, Shigella, Klebsiella spp., and Acinetobacter baumannii, but Burkholderia spp., Serratia, and Proteus spp. are resistant.
- Naturally resistant gram-negative bacteria, such as Proteus mirabilis and Burkholderia cepacia, show the complete substitution of their lipid phosphate by ethanolamine or aminoarabinose.
- Absorption- the possibility of systemic absorption should always be borne in mind when treating patients by the inhalation route.
- Distribution- The volume of distribution of colistin in healthy subjects is low and corresponds approximately to the extracellular fluid. The volume of distribution is relevantly enlarged in critically ill subjects.
- Elimination- It is estimated that approximately 30% of colistimethate sodium is converted to colistin in healthy subjects. Its clearance is dependent on creatinine clearance and as renal function decreases, a greater portion of colistimethate sodium is converted to colistin.