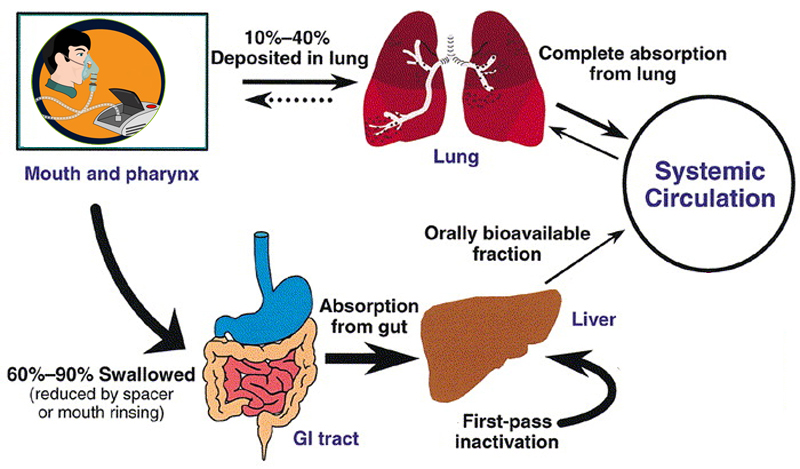
1. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20929lbl.pdf
2. Allen B. et.al. Inhaled corticosteroids. Journal of Allergy and Clinical Immunology
3. Bloom CI, Drake TM et.al; ISARIC investigators. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021 Jul;9(7):699-711. doi: 10.1016/S2213-2600(21)00013-8. Epub 2021 Mar 4. PMID: 33676593; PMCID: PMC8241313.
4.https://www.who.int/news-room/questions-and-answers/item/chronic-respiratory-diseases-asthma
5. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020929s052lbl.pdf
6. van Essen-Zandvliet EE et.al. Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. 1992
7. https://doi.org/10.1159/000430443
8. Yi Cai and Anna Meyer,Pediatric Infectious Disease.
9. Allison Gates, PhD; David W. Johnson, MD; Terry P. Klassen, MD. Glucocorticoids for Croup in Children
10. Russell K, Wiebe N, Saenz A, et al. Glucocorticoids for croup. Cochrane .DatabaseSyst Rev, 2004;CD001955.
11. https://www.nejm.org/doi/full/10.1056/nejm199408043310501
12. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020929s052lbl.pdf
13. Bloom CI, Drake TM et.al; ISARIC investigators. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021 Jul;9(7):699-711. doi: 10.1016/S2213-2600(21)00013-8. Epub 2021 Mar 4. PMID: 33676593; PMCID: PMC8241313.
14. Husby, S., Agertoft, L., Mortensen, S., & Pedersen, S. (1993). Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Archives of disease in childhood, 68(3), 352–355. https://doi.org/10.1136/adc.68.3.352
15.Andersson, M., Lindqvist, N., Svensson, C., Ek, L., &Pipkorn, U. (1993). Dry powder inhalation of budesonide in allergic rhinitis. Clinical otolaryngology and allied sciences, 18(1), 30–33. https://doi.org/10.1111/j.1365-2273.1993.tb00805.x
16. O'Donnell, S., &O'Morain, C. A. (2010). Therapeutic benefits of budesonide in gastroenterology. Therapeutic advances in chronic disease, 1(4), 177–186. https://doi.org/10.1177/2040622310379293
17.Kalola UK, Ambati S. Budesonide. [Updated 2021 Dec 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563201/