Overview of Dydroboon
- Progesterone is a steroid hormone of essential role in reproduction, preparation and maintenance of pregnancy1.
- Progesterone deficiency is managed by administering oral progesterone, oral natural micronized progesterone, micronized intravaginal progesterone, intramuscular synthetic progesterone2.
- Dydroboon contains dydrogesterone, a synthetic progestogen, that has high affinity for progesterone receptors, low affinity for androgen receptors, and no affinity for oestrogenic receptors2.
Indications
Position Statements for Dydrogesterone

- Dydrogesterone is known to have immunomodulatory properties in early pregnancy 2
- Dydrogesterone is thus beneficial in women presenting with a clinical diagnosis of threatened and recurrent miscarriage2
Federation of Obsteric and Gynaecological Societies of India (FOGSI)

- For prolonged treatment, dydrogesterone is preferred to other progestogens because of its less negative effect on lipid metabolism and less androgenic effects 3
European Society of Human Reproduction and Embryology (ESHRE)

- Dydrogesterone treatment is significantly associated with fewer miscarriages, less severe pain, and higher rate of pregnancy at 20 weeks gestation in women with threatened miscarriages 4
National Institute for Health and Clinical Excellence
(NICE)
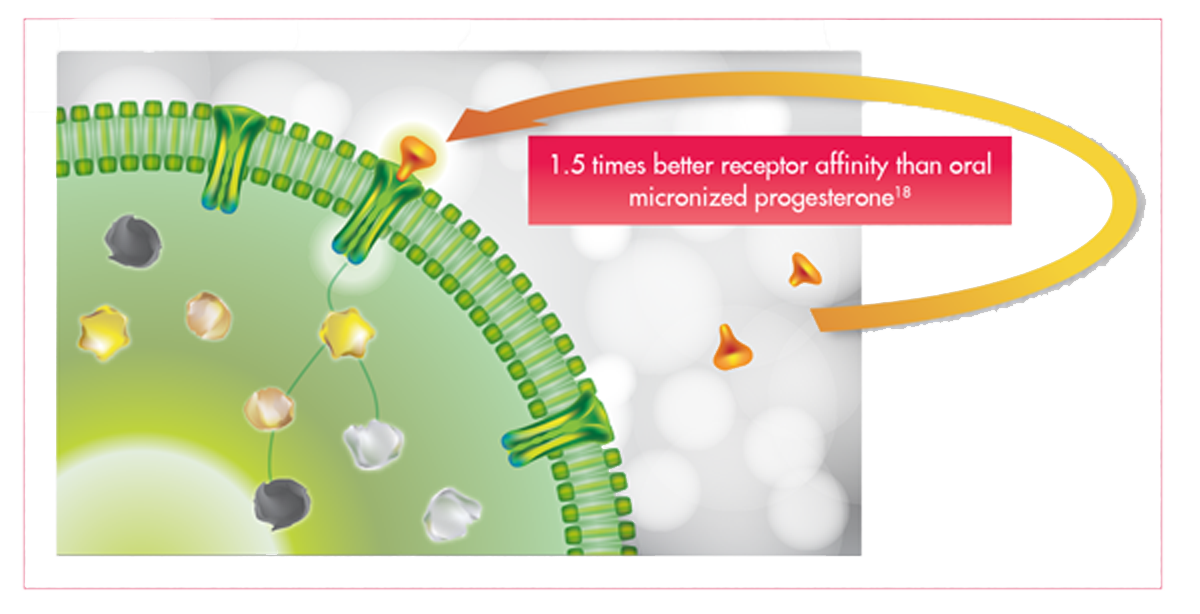
How is dydroboon better than natural micronized progesterone (NMP) ?
Dydrogesterone offers 10
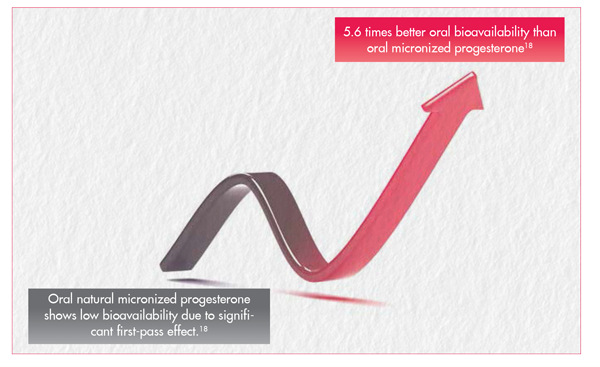
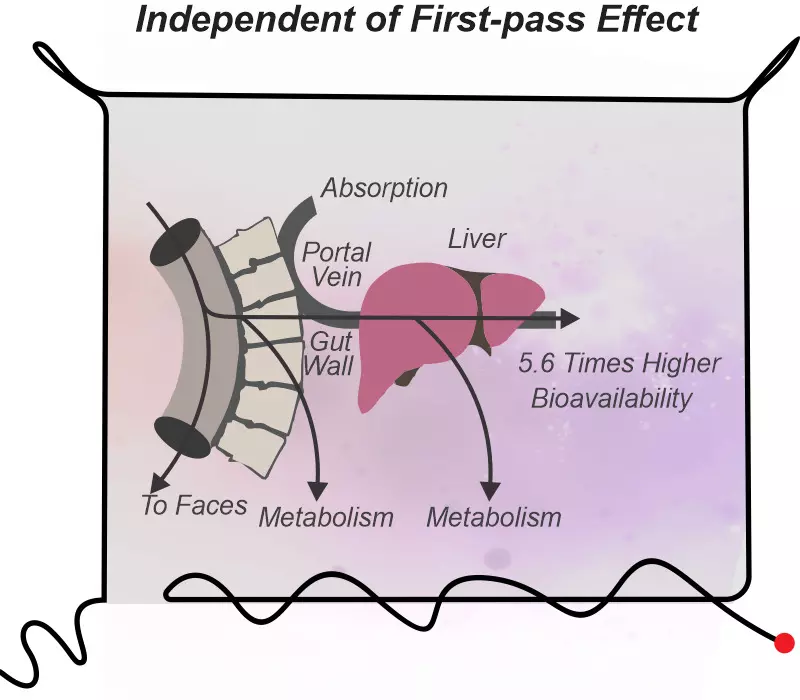
- 5.6 times higher oral bioavailabilty
- 50% more receptors affinity
- 6 times quick onset of action
- For secure pregnancy NMP may not be optimal in recurrent pregnancy loss, threatened abortion and luteal phase deficiency
- Oral NMP has just 6-8% absolute bioavailability & has increased risk of intrahepatic cholestasis 11
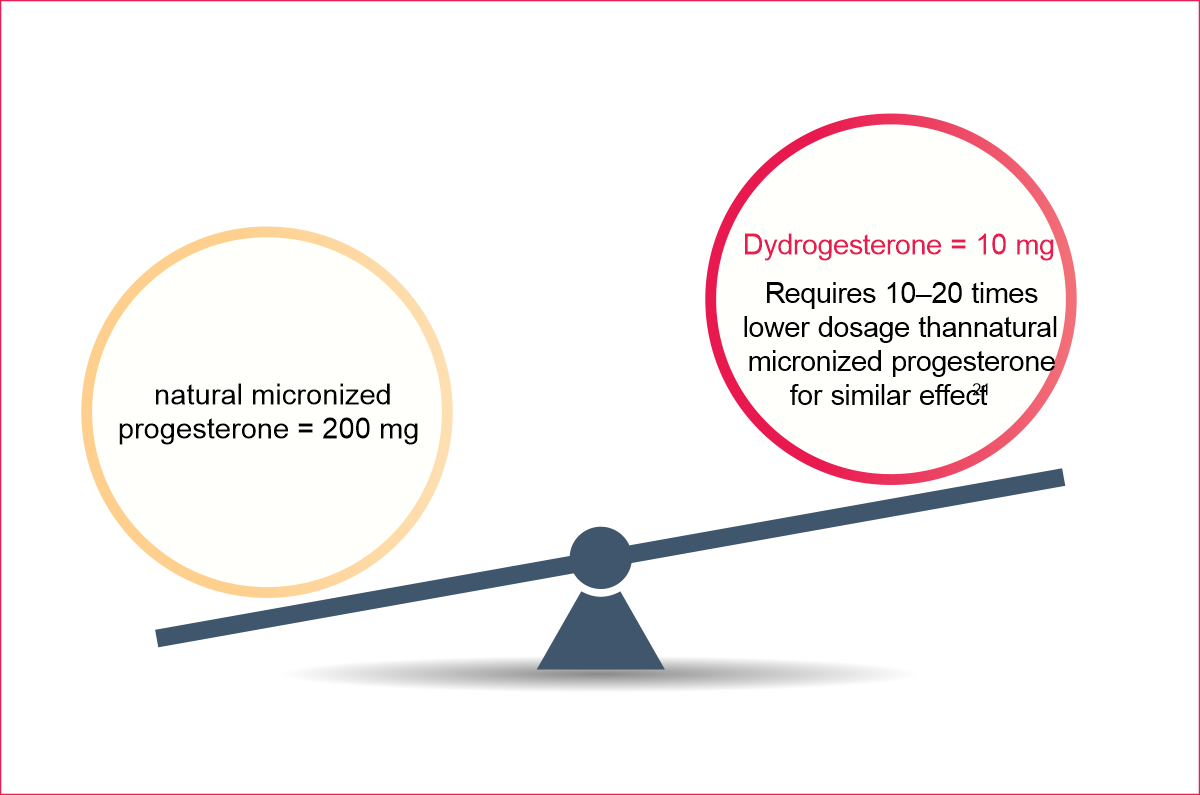
10-15 Times lesser dosage requirement 11
Dydroboon in threatened Abortion and Miscarriage12
Incidence of miscarriage in both study groups
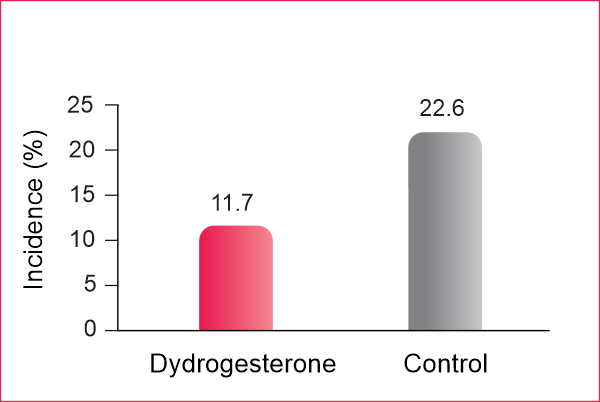
- Incidence of miscarriage was significantly lower in the oral dydrogesterone group than in the control group (11.7% versus 22.6%)
- Oral dydrogesterone effectively prevents miscarriages in pregnant women experiencing threatened abortion.
- Dydrogesterone significantly reduces miscarriage by 47%
- Dydrogesterone reduces the incidence of pregnancy loss in threatened abortion during the first trimester in women without a history of recurrent abortion
Dydroboon in Pregnancy related disorders and complications13
On-going pregnancy rate with oral dydrogesterone and VP gel
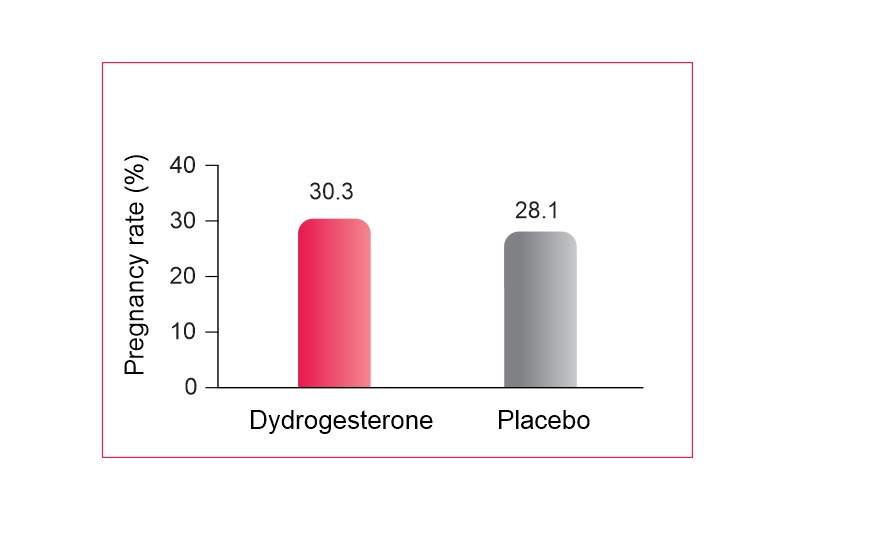
- Comparable on-going pregnancy rates were observed between the oral dydrogesterone and VP gel groups
- Oral dydrogesterone group recorded significantly higher overall satisfaction and tolerability than the VP gel group
- Vaginal bleeding, interference with coitus, and vaginal irritation and discharge occurred significantly more in the VP gel group versus the dydrogesterone group
Dydroboon in Preterm birth 14
No. of patients with preterm delivery
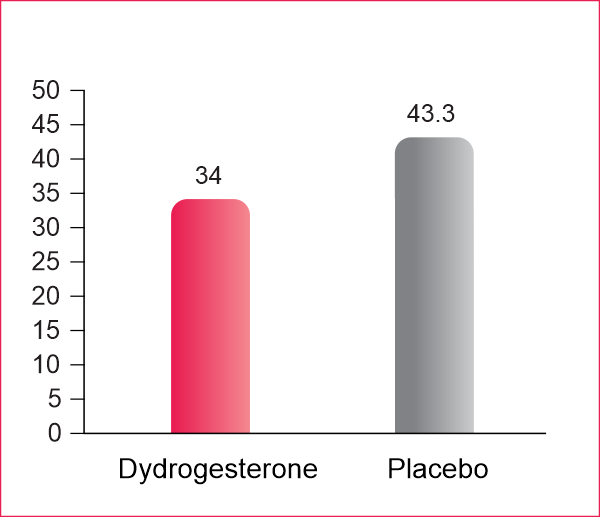
- 34.0% in the dydrogesterone group delivered before the 37th week of gestation, whereas the 43.3% of pre-term delivery was registered in the non-progesterone group
- The length of gestation significantly increased in dydrogesterone treated women at risk of preterm delivery.
Dydroboon in Menstrual Disorders 15
Number of patients with withdrawal bleeding
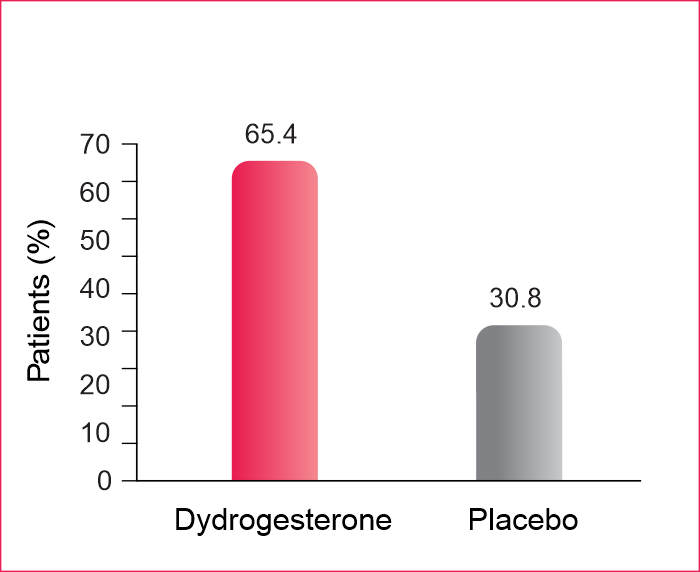
- The number of women with withdrawal bleeding during the first cycle was twice as high in the dydrogesterone group as in the placebo group (65.4% vs. 30.8%)
- Superiority of dydrogesterone was also observed for regularity of bleeding over the six cycles.
- Dydrogesterone is significantly superior to placebo in inducing withdrawal bleeding, and maintaining regular bleeding, in women with secondary amenorrhea and normal estrogen levels
Dosage
| Indications | Dosage |
|---|---|
| Dysmenorrhea | 10 mg twice daily |
| Secondary amenorrhea | |
| Dysfunctional uterine bleeding (for 5 to 7 days) | |
| Irregular cycles and habitual abortion (until 20th week of pregnancy) | |
| Premenstrual syndrome | |
| Infertility (due to luteal insufficiency) for at least six consecutive cycles | 10 mg once daily |
| Hormone replacement therapy | |
| Threatened abortion | 40 mg immediately, then 10 mg every 8 hours until symptoms remit |
| Endometriosis | 10 mg twice or thrice daily from day 5 to 25 or continuously |
Pharmacokinetics of Dydrogesterone
Absorption 5
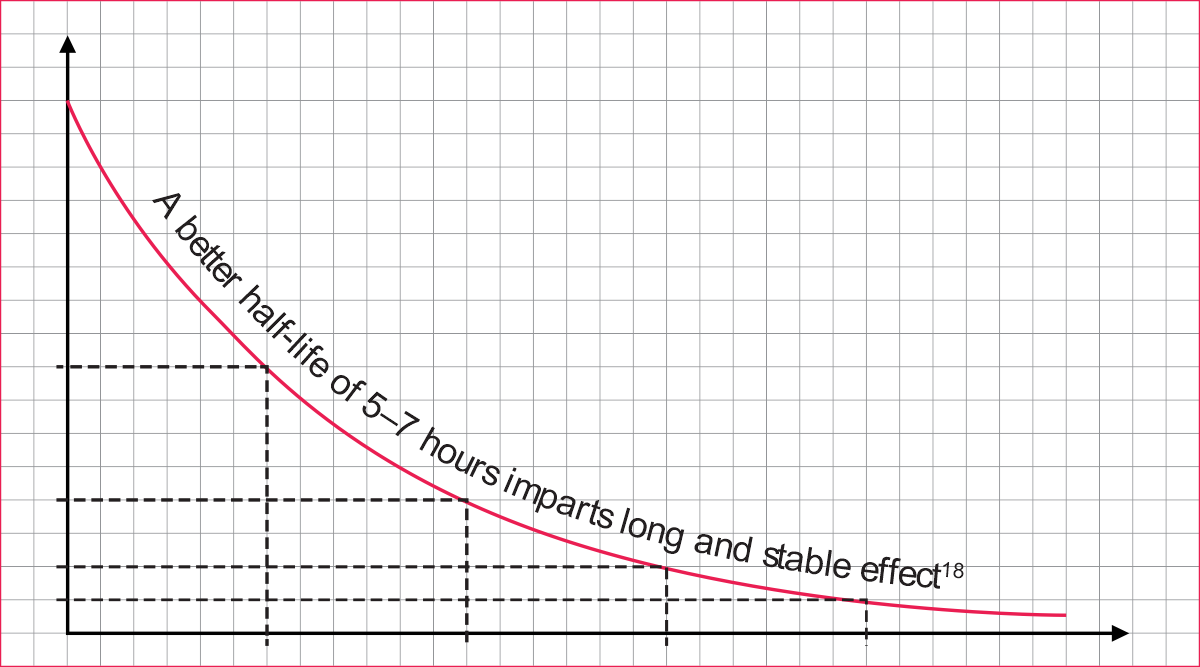
- Gets rapidly absorbed
- Attains maximum plasma levels in 0.5–2.5 hours
- Shows mean half-life of 5–7 hours
Distribution6
- Mainly binds to transport proteins and tissue steriod receptors
Metabolism 7
- Metabolizes completely
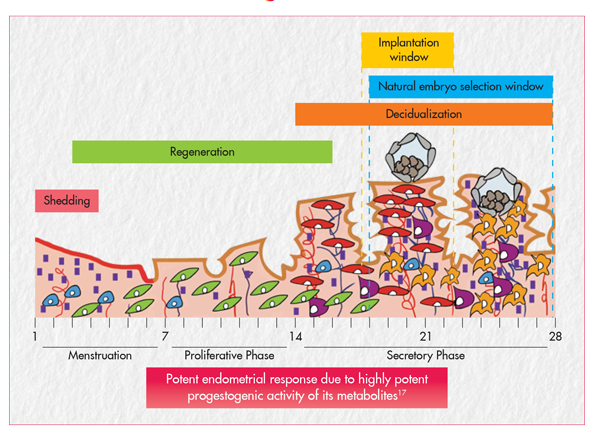
- Gets converted to DHD, which is a potent progestogen
Excretion 8
- Gets completely excreted within 72 hours
Frequently Asked Questions
- 1. What is Dydroboon ?
Dydroboon is a synthetic progestogen, that has high affinity for progesterone receptors, low affinity for androgen receptors, and no affinity for oestrogenic receptors.
- 2. What is Dydroboon indicated for ?
Dydroboon is indicated in the following conditions:
- Amenorrhea (primary and secondary)1
- Endometriosis
- Prevention of preterm birth and miscarriages
- Abnormal uterine bleeding 1
- Sub-mucous fibroids 1
- Uterine cancer 1
- Recurrent and threatened abortions
- Irregular menstrual cycle
- Infertility
- 3. What are the clinical pharmacokinetics of Dydroboon ?
The clinical pharmacokinetics of Dydroboon is as follows:
- Absorption: Dydroboon gets rapidly absorbed. It attains maximum levels in 0.5-2.5 hours and a half-life of 5-7 hours.
- Distribution: Mainly binds to transport proteins and tissue steroid receptors.
- Metabolism: It metabolizes completely by converting it into DHD which is a potent progesterone.
- Excretion: It gets completely excreted within 72 hours.
- 4. What is the mechanism of action of Dydroboon ?
Dydroboon is an orally active progestogen which produces a complete secretory endometrium in an estrogen-primed uterus thereby providing protection against the increased risk for endometrium hyperplasia and/or carcinogenesis induced by estrogens. It is indicated in all cases of endogenous progesterone deficiency. Dydroboon has no estrogenic, no androgenic, no thermogenic, no anabolic and no corticoid activity.
- 5. What are the recommended dosage for Dydroboon ?
The recommended dosage of Dydroboon is as follows:
- Dysmenorrhea, secondary amenorrhea, dysfunctional uterine bleeding (for 5 to 7 days), irregular cycles and habitual abortion (until 20th week of pregnancy), and premenstrual syndrome: 10 mg twice daily
- Infertility (due to luteal insufficiency) for at least six consecutive cycles: 10 mg once daily
- Threatened abortion: 40 mg immediately, then 10 mg every 8 hours until symptoms remit
- Endometriosis : 10 mg twice or thrice daily from day 5 to 25 or continuously
- Hormone replacement therapy : 10 mg once daily
- 6. What are the side effects of Dydroboon ?
The common side effects of Dydroboon includes:
- Breast pain
- Vaginal bleeding
- Stomach Pain
- Menstrual disorder
- Nausea
- Vomiting
- Abdominal Pain
- Headache
- Drowsiness
- Itching
- Cough
- 7. What are the common adverse effects of Dydroboon?
Most commonly reported adverse effects in patients who were treated with Dydroboon without use of estrogen were metrorrhagia, painful/sensitive breasts, and migraine/headache.
- 8. What are the contraindications of Dydroboon ?
- Vaginal bleeding, where the cause has not been established
- Presence of serious liver disorders, until the liver function values return to normal
- Contraindications for the use of estrogens in combination with Dydroboon
- Known hypersensitivity to Dydroboon or any of the excipients
- Known or suspected sex hormone-dependent malignancies
- 9. What are the special warnings and precautions to be taken while using Dydroboon? ?
Before initiating Dydroboon in dysfunctional uterine bleeding, an organic cause should be excluded. Breakthrough bleeding and spotting may occur during the first months of treatment. If breakthrough bleeding and spotting continue to occur when treatment has already been underway for some time, or continue when treatment is discontinued, the cause of this should be ascertained, if necessary by taking an endometrial biopsy to exclude malignancy of the endometrium. Dydroboon treatment should be stopped if one of the disorders occurs during use for the first time or gets worse such as i) exceptionally severe headache, migraine, or symptoms that may indicate cerebral ischemia ii) marked increase in blood pressure iii) occurrence of venous thromboembolism.
In cases of habitual or threatened abortion, the fetus viability should be ascertained, and it is necessary to monitor during treatment whether the pregnancy is still progressing and whether embryo is still alive. The conditions for which monitoring are necessary:
- It is known that the following rarely occurring conditions may be affected by sex hormones and may arise or get worse during pregnancy or during the use of sex hormones: cholestatic icterus, herpes gestations, severe pruritus, otosclerosis, and porphyria.
- Patients with a history of depression must be carefully monitored; if severe depression recurs, treatment with dydrogesterone must be stopped.
- 10. What is the safety profile of Dydroboon in mother and fetus ? No significant safety concerns related with Dydroboon had been observed for either mother or the fetus.
- Preclinical studies revealed no mutagenic,teratogenic, or carcinogenic potential
- No clinical evidence for congenital malformations during pregnancy
- No change in coagulation parameters, non-hepatotoxic and no thermogenic properties
- Low incidence of subjective side effects, such as headache and mood changes
- No change in body weight and blood pressure
- Unlike other synthetic progestins, does not affect blood lipids and carbohydrate metabolism
- 11. What happens with the overdose of Dydroboon ?
Limited data are available with regard to overdose of Dydroboon in humans. There are no specific antidotes and treatment should be symptomatic. This information is also applicable for overdose in children.
- 12. What is the suggested storage conditions for Dydroboon ?
Dydroboon tablet should be kept at room temperature between 68ºF and 77ºF (20ºC and 25ºC).