
Want to Know More about Molnupiravir Check out this Video.
Molulife in Media
What is Molnupiravir?
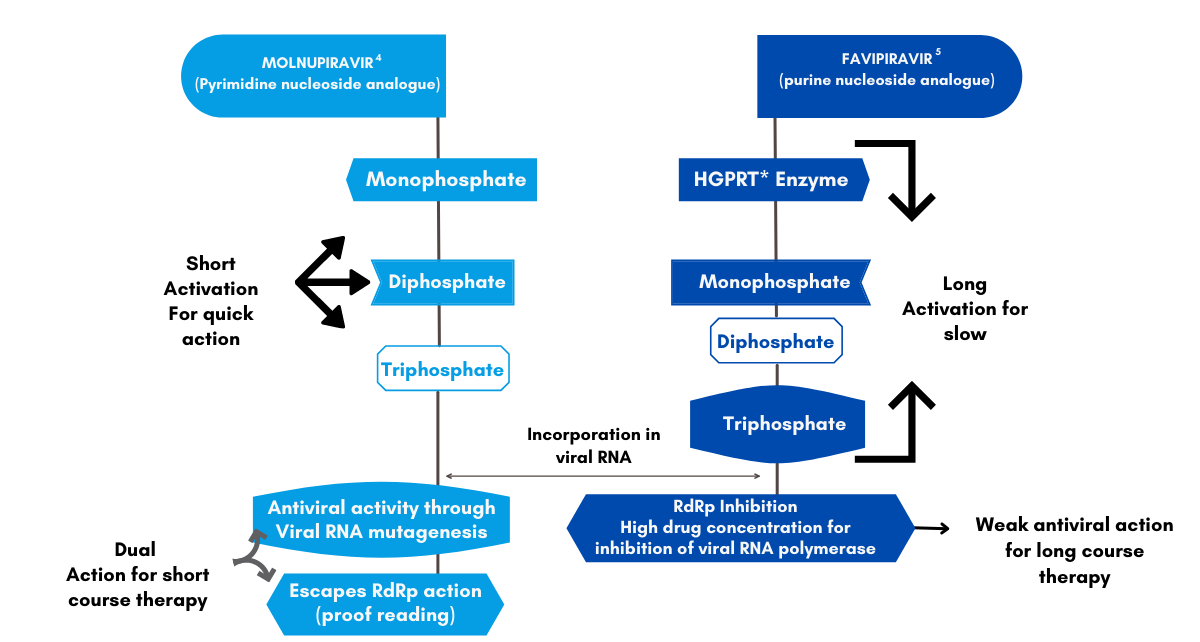
- Molnupiravir the pro drug of the active antiviral ribonulceoside analog β-D-N4-hydroxycytidine (NHC; EIDD-1931), has activity against number of RNA viruses, including SARS-CoV-2 that causes COVID-19.
- Molnupiravir is indicated for treatment of mild to moderate coronavirus disease (COVID-19) in adults with a positive SARS-COV-2 diagnostic test and who have at least one risk factor for developing severe illness1.
Molnupiravir Approved by US FDA for Treatment of Mild to Moderate COVID-19 in High Risk Adults*

Age > 60 years

Cardiovascular disease, hypertension, and CAD
DM (Diabetes mellitus) and other immunocompromised states
Cerebrovascular disease
Obesity
Chronic lung/kidney/liver disease
Efficacy across Gamma , Delta Mu

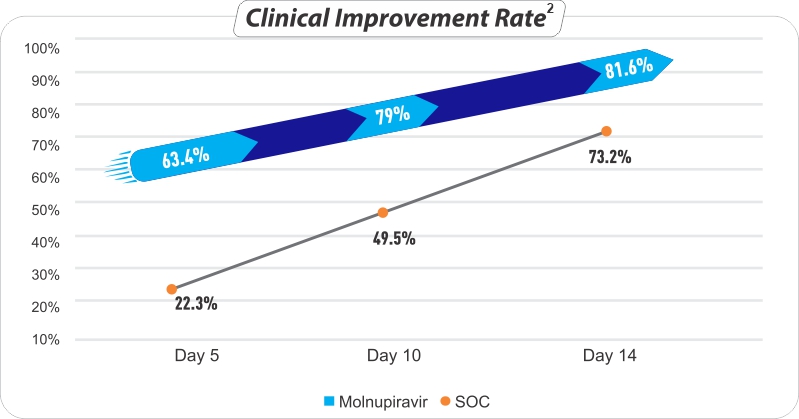
- Fastens Clinical Recovery2
- Reduces COVID-19 symptoms
Within ≤ 5 days - Higher RT- PCR negativity rate in patients after completion of 5 Days treatment2
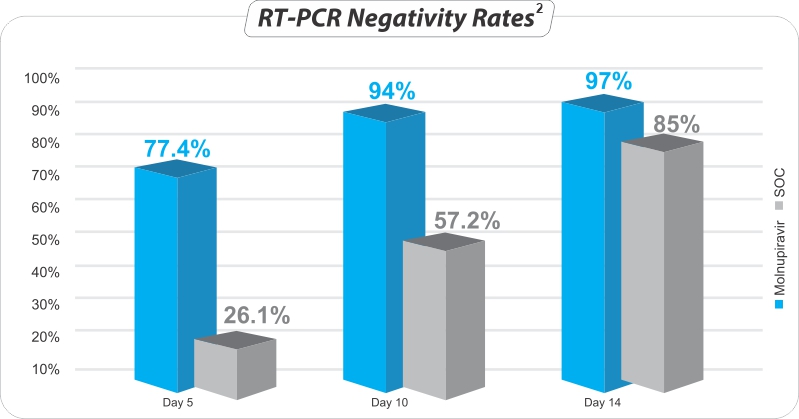

- Reduces relative risk of all cause Hospitalisation or death by 30%3
- Minimises comparative risk of COVID-19 related death by 89%3,#
- Clinical improvement in 82% patients2
Molnupiravir Over Favipiravir
The phase 2 portion of the outpatient study (P002, MOVe-OUT) was Designed to Facilitate Dose Selection
- Adults (≥18 years) with mild/moderate COVID-19
- Symptomatic + positive SARS-CoV-2 test result ≤7 days prior to study entry
- Symptom duration ≤7 days at entry
- Randomized, double-blind
- 200, 400, 800 mg molnupiravir vs placebo BID for 5 days (~75 per arm)
- Randomization stratified by symptom duration at entry and increased risk status
- Primary Endpoint: Hospitalization or death through Day 29
- Other endpoints to inform dose selection: viral load, infectivity, and viral nucleotide substitution analysis

- Number of sites who screened in Phase 2: 58
- Number of countries: 12(including the United States)
- Total Phase 2 Recruitment: 303
The safety and tolerability in patients with mild to moderate COVID-19 were evaluated. Twice daily oral doses of 200 mg, 400 mg and 800 mg molnupiravir for 5 days.
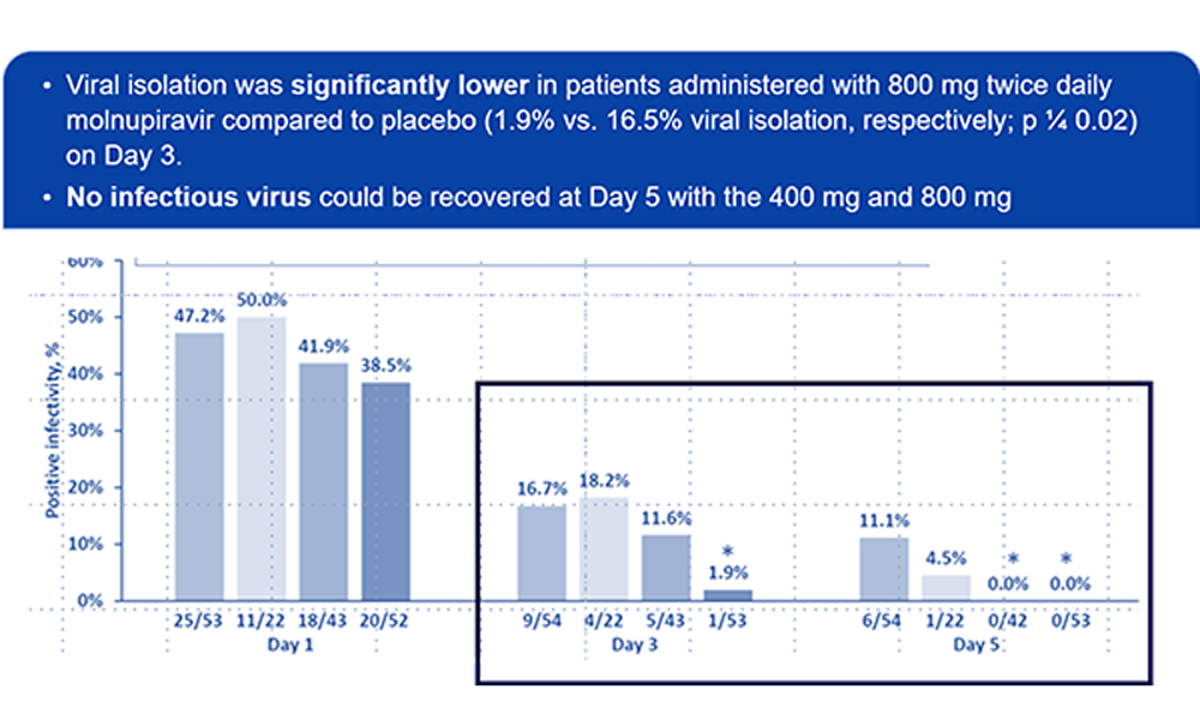
The phase 3 double-blind, randomized study (MOVe-OUT) to assess efficacy and safety of molnupiravir in 1850 non-hospitalized adult (18 years or older) participants with COVID-19
Laboratory-confirmed SARS-CoV-2 infection ≤5 days prior to randomization Onset of COVID-19 signs/symptoms ≤5 days prior to randomization.
All at increased risk for severe illness from COVID-19>60 years of age, active cancer, CKD, COPD, obesity (BMI ≥30), serious heart conditions (CAD, heart failure, cardiomyopathies), diabetes mellitus.
Unvaccinated against SARS-CoV-2N=1550.
Molnupiravir 800 mg every 12 hours for 5 days vs placebo, randomized 1:1.
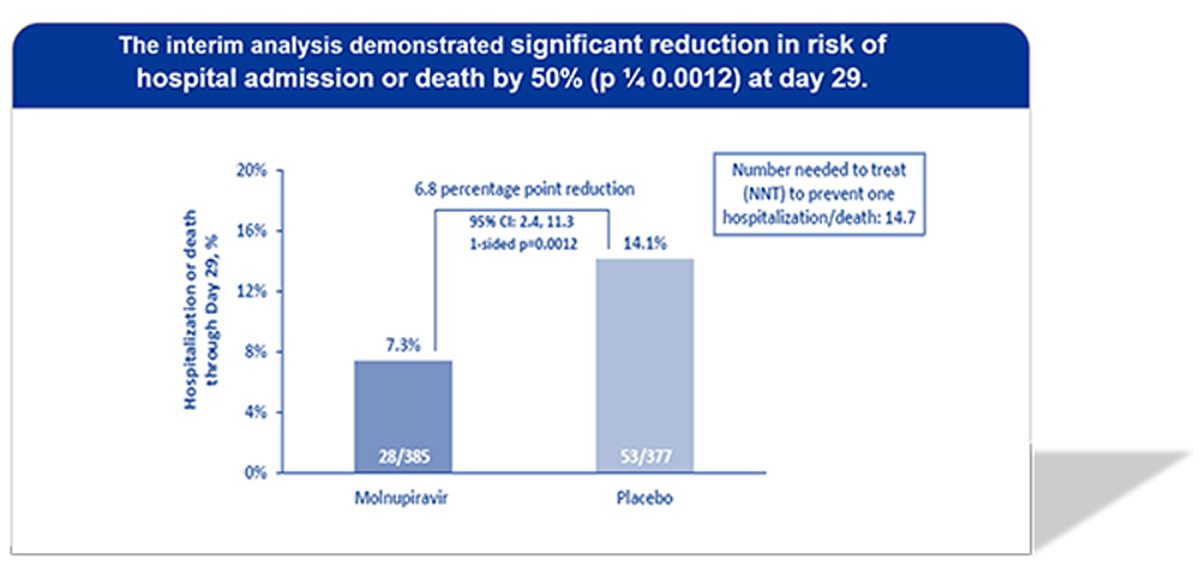
Primary: All-cause hospitalization (≥24 hours) or death through Day 29.
Secondary: Improvement/progression of patient-reported signs/symptoms of COVID-19 through Day 29 WHO 11-point ordinal scale measuring COVID-19 severity at pre-specified time points .
Recommendations

Medicines and Healthcare products Regulatory Agency (MHRA)recommends Molnupiravir for patients who have mild to moderate COVID-19 and at least with one risk factor obesity, older age (>60 years), diabetes mellitus, or heart disease.

U.S. Food and Drug Administration issued an emergency use authorization (EUA) on molnupiravir for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate.

In India the Drugs Controller General of India (DCGI), has approved molnupiravir for treatment of adult patients with Covid-19, with SpO2 > 93% and who have high risk of progression of the disease including hospitalization or death.

When to use Molnupiravir?
Within 5 days of symptom onset (provided a statistically significant reduction in the rate of Hospitalisation or death in the population ) Molnupiravir is not authorized for use for longer than five consecutive days.6
Dose and Duration?
Molnupiravir 800mg (200mg Cap/Tap) every 12 hours for 5 days, with or without food
*Treatment should start with 5 days of symptom onset.7
How to use?*
- Molnupiravir is available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset.
- Molnupiravir is not authorized for use in patients younger than 18 years of age because molnupiravir may affect bone and cartilage growth.
Benefits of Molnupiravir8
- Broad spectrum oral antiviral agent.
- Required to be given only for short duration (5 days) .
- Better patient compliance and easier to administer in OPD settings.
- Well tolerated and safe without any major adverse effects.
- Reduces COVID-19 symptoms within ≤5 days.
- A relative risk reduction of 52% (95% CI 33%, 80%) in hospitalization or death through Day 29 compared to placebo.
- Drastically decreases disease progression by reducing hospitalization and/or death.
- Difficult for virus to evolve resistance to molnupiravir.
- 80% of the cases analysed have prevalent gamma, mu and delta SARS-CoV-2 variants.
- Or Efffective against Variants – Gamma, Mu, Delta.
Frequently Asked Questions
- What is Molulife-200 Capsule?
Molulife is the brand of Molnupiravir (EIDD-2801/MK-4482), the pro drug of the active antiviral ribonulceoside analog β-D-N4-hydroxycytidine (NHC; EIDD-1931), which has activity against number of RNA viruses, including SARS-CoV-2 that causes COVID-19.
- Is it safe to use Molulife-200 Capsule in COVID-19?
Molulife (Molnupiravir) offers a favourable tolerability and safety profile. The most frequent adverse events (reported in ≥1% of subjects) reported during treatment were diarrhoea, nausea, dizziness and headache all of which were Grade 1 (mild) or Grade 2 (moderate).
- Are there any specific cautions associated with the use of Molulife 200 Capsule?
- Molulife (Molnupiravir) usage is limited to non-pregnant adults only. No dosage adjustments are indicated in elderly, renal impairment, and hepatic impairment.
- The safety and efficacy of Molulife (Molnupiravir) in patients below 18 year of age have not been established.
- What if I miss the dose of Molulife?
- If the patient misses a dose of Molulife within 10 hours of the time it is usually taken, the patient should take as soon as possible and resume the normal dosing schedule.
- If a patient misses a dose by more than 10 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time.
- The patient should not double the dose to make up for a missed dose.
- What are the instructions for Pregnant and Lactating mothers regarding usage of Molulife?
- Pregnancy: There are no data from the use of Molulife in pregnant women. Studies in animals have shown reproductive toxicity.
- Molulife is not recommended during pregnancy. Women of childbearing potential should use effective contraception of the duration of treatment and for 4 days after the last dose of Molulife.
- Breast-feeding: It is unknown whether Molnupiravir or any of the components of Molnupiravir are present in human milk, affect human milk production, or have effect on the breastfed infant. Animal lactation studies with Molnupiravir have not been conducted.
- Based on the potential for adverse reactions on the infant from Molulife, breast-feeding is not recommended during treatment and for 4 days after the last dose of Molulife.
- For how long I should take Molulife and what is the dosage?
- The recommended dose of Molulife is 800 mg (four 200 mg capsules) taken orally every 12 hours for 5 days.
- The safety and efficacy of Molnupiravir when administered for periods longer than 5 days have not been established.
- What are the indications for Molulife Capsule?
- Molulife is indicated for treatment of mild to moderate coronavirus disease (COVID-19) in adults with a positive SARS-COV-2 diagnostic test and who have at least one risk factor for developing severe illness.
- One or more pre-defined risk factors for disease progression in COVID-19 are –
- Age ≤60 years
- Diabetes
- Obesity (BMI>30)
- Chronic kidney disease
- Serious heart conditions
- Chronic obstructive pulmonary disease
- Active cancer

















