Heart Failure - Overview
Heart failure is a progressive chronic syndrome characterized by decrease in functional status and quality of life1.
The burden of heart failure has increased to an estimated 23 million people, worldwide. In India, the prevalence was estimated to be around 1.2/1000 people in the INDUS study....
As prevalence rates of heart failure are high and expected to rise in the near future because of improved survival from acute cardiac events and the aging of the general population, thus HF management has come to the front-stage of non-communicable disease management programmes.
Once developed, heart failure has a 1-year mortality rate of 7.2% and a 1-year hospitalization rate of 31.9% in patients with chronic heart failure, and in patients hospitalized for acute heart failure, these rates increase to 17.4% and 43.9%2.
The pathophysiology of heart failure involves a maladaptive response during which the renin-angiotensin-aldosterone system (RAAS) is activated3.
RAAS activation leads to vasoconstriction, hypertension, increased aldosterone levels, increased sympathetic tone, and eventually, cardiac remodeling, all of which are detrimental to the progression of the disease3.
Indication

Heart Failure
To reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
Important update
Understanding ARNI in Hypertension
Understanding Heart Failure in India
Guidlines defined for the treatment of Heart Failure
Quick Review- Role of ARNI in Heart Failure final
Decoding the clinical Evidence for Sacubitril Valsartan in Heart Failure
The Drug Review Sacubitril Valsartan
John McMurray
BHF Cardiovascular Research Centre, University of Glasgow & Queen Elizabeth University Hospital, Glasgow, Scotland, UK
Dr. Partho P Chowdhury
MD (DELHI UNIVERSITY), DM (IPGMER,KOL) Consultant Interventional Cardiologist
Specialist in Radial Angioplasty, Complex Angioplasty, Pacemaker, ICD, CRT Implantation, Device Closure
Meditrina Hospital, Jharkhand
Professor Dr. J.C Mohan
MBBS, MD (General Medicine) DM (Cardiology) M.NAMS FACC (Fellow American College of Cardiology) FASE (Honorary Fellow of American Society of Echocardiography)
FESC (Fellow of European Society of Cardiology) Jaipur Golden Hospital, Delhi
Dr. Armendra Kumar Pandey
MBBS, DNB (Medicine) FNIC, DNB (Cardilogy)
Consultant-Cardiology Dharamshila Narayana Superspeciality Hospital
Dr. Dilip Kumar
MBBS, MD, DM (Card) FRCP (GLASG), FHRS, FSCAI, FESC, IBHRE, CCDS Chief Academic Co-ordinator Medica Institute of Cardiac Sciences Kolkata
Dr. Animesh Agarwal
MD, DM (Cardiology), AFESC International Associate American College of Cardiology
Senior Consultant & HOD Department of Cardiology Jindal Institute of Medical Sciences, Haryana
Dr. Harpreet Singh Gilhotra
DM, FESC Director Cardiology
Sri Guru Harkrishan Sahib (C) Eye Hospital Trust, Sohan (Sohana Hospital)
Dr. Amit Handa
Consultant- Cardiologist
MD (Med.), DM (Cardiology) Ivy Multi Speciality Hospital Punjab
Dr. Niroj Kumar Mishra
M.D. (Medicine) Clinical Director
(AN ISO:9001:2008 CERTIFIED HOSPITAL) KAR CLINIC & HOSPITAL PVT.LTD., Bhubaneswar
Dr. Nitin Tiwari
M.B.B.S (Gold Medallist) D.M.(Card), D.N.B.(Card), M.D. (Card), D.N.B (Med.), M.N.A.M.S., M.A.P.S.I.C., F.I.A.M.S.
FIC (France), FIC (Germany), FESC (Europe), FACC (USA) Interventional Cardiologist WOCKHARDT HEART HOSPITAL, Nagpur
Dr. Idris Ahmed Khan
MD, DM (Card, PGI Chandigarh)
Consultant Interventional Cardiologist BOMBAY HOSPITAL, INDORE
Dr. Johann Christopher
MD, DNB (Cardiology)
Consultant Cardilogist Division of Cardiac Imaging CARE HOSPITALS, CARE OUTPATIENT CENTRE Hyderabad
Dr. C.K. Ponde
Consultant Cardiologist M.D. (Gen.Med), D.M (Card),
D.N.B. (Card) FACC (USA), FSCAI (USA) FCSI, FISE, FICC, FIAE
Prof Dr Satyanarayan Routray
MBBS, MD (MEDICINE), DM (CARDIOLOGY), FICC,FCSI Professor and HOD
SCB Medical College & Hospital Cuttack
Dr J.S Hiremath
DM (Cardiology), DNB (Cardiology) Fellow of American College of Cardiology Director: Cath Lab, Ruby Hall Clinic
Chief Cardiologist, Hearty Transplant Department, Ruby Hall Clinic, Pune
About Azmarda
Azmarda contains Sacubitril/Valsartan, the first agent to be approved in a new class of drugs called angiotensin receptor neprilysin inhibitor (ARNI)3.


How Azmarda Works?
Sacubitril acts as a neprilysin inhibitor by preventing the breakdown of natriuretic peptides. This leads to a prolonged duration of the favorable effects of these peptides. Valsartan is an angiotensin receptor blocker, and it works by blocking the renin-angiotensin-aldosterone system3.
Composition
Pharmacokinetics of Azmarda4
Absorption
Following oral administration, Azmarda dissociates into sacubitril, which is further metabolized to sacubitrilat, and valsartan, which reach peak plasma concentrations in 0.5 hours, 2 hours, and 1.5 hours, respectively.
The oral absolute bioavailability of sacubitril and valsartan is estimated to be ≥ 60% and 23%, respectively. Azmarda can be administered with or without food.
Distribution
Azmarda is highly bound to plasma proteins (94% - 97%). Based on the comparison of plasma and CSF exposures, sacubitrilat does cross the blood brain barrier to a limited extent (0.28%).
Azmarda has an apparent volume of distribution ranging from 75L to 103L.
Biotransformation
Sacubitril is readily converted to sacubitrilat by esterases; sacubitrilat is not further metabolized to a significant extent.
Valsartan is minimally metabolized, as only about 20% of the dose is recovered as metabolites.
Elimination
Following oral administration, 52 to 68% of sacubitril (primarily as sacubitrilat) and ~13% of valsartan and its metabolites are excreted in urine; 37 to 48% of sacubitril (primarily as sacubitrilat), and 86% of valsartan and its metabolites are excreted in feces. Sacubitril, sacubitrilat, and valsartan are eliminated from plasma with a mean elimination half-life (T1/2) of approximately 1.43 hours, 11.48 hours, and 9.90 hours, respectively.
Clinical Evidences
Clinical Evidences
Dosage
- The recommended starting dose of AZMARDA is 100 mg twice daily.
- A starting dose of 50 mg twice daily is recommended for patients not currently taking an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB), and should be considered for patients previously taking low doses of these agents.
- The dose of AZMARDA should be doubled every 2-4 weeks to the target dose of 200 mg twice daily, as tolerated by the patient.
Questions and Answers
- 1. What is Azmarda (Sacubitril Valsartan)?
Azmarda is a brand name for the drug combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. It belongs to the angiotensin-neprilysin inhibitors (ARNI) class of drugs.
- 2. What is Azmarda (Sacubitril Valsartan) indicated for?
Azmarda containing Sacubitril/ Valsartan is indicated in adults with long-term heart failure who have symptoms of the disease to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
- 3. How does Azmarda (Sacubitril Valsartan) work?
Azmarda containing Sacubitril/ Valsartan exhibits the novel mechanism of action of an angiotensin receptor neprilysin inhibitor (ARNI) by simultaneously inhibiting neprilysin (neutral endopeptidase; NEP) via sacubitril, the active metabolite of the prodrug sacubitril, and by blocking the angiotensin II type-1 (AT1) receptor via valsartan. The complementary cardiovascular benefits and renal effects of Azmarda in heart failure patients are attributed to the enhancement of peptides that are degraded by neprilysin, such as natriuretic peptides (NP), by sacubitril and the simultaneous inhibition of the deleterious effects of angiotensin II by valsartan.
Sustained activation of the renin-angiotensin-aldosterone system results in vasoconstriction, renal sodium and fluid retention, activation of cellular growth and proliferation, and subsequent maladaptive cardiovascular remodeling. Valsartan inhibits detrimental cardiovascular and renal effects of angiotensin II by selectively blocking the AT1 receptor, and also inhibits angiotensin II-dependent aldosterone release - 4. What are the dosage forms and strengths of Azmarda (Sacubitril Valsartan)?
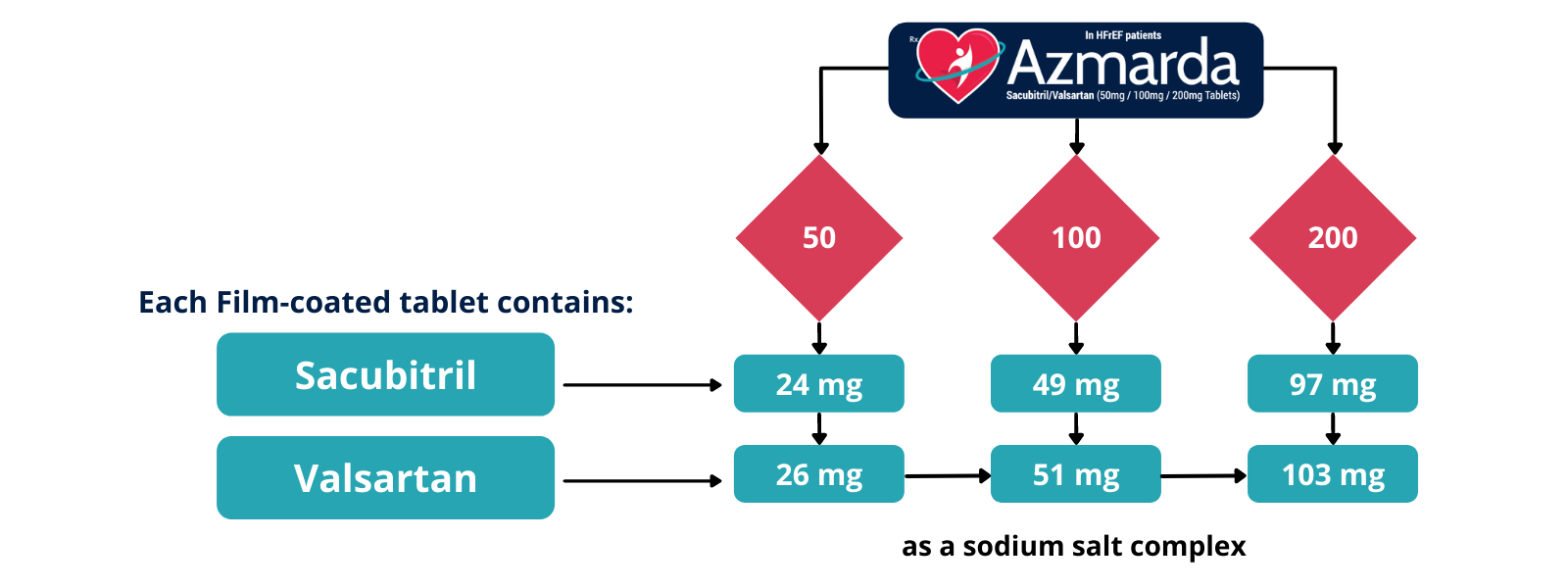
Azmarda containing Sacubitril/ Valsartan is available in different forms as Azmarda 50 mg, Azmarda 100 mg, and Azmarda 200 mg. It is supplied as unscored, ovaloid, film-coated tablets in the following strengths:
Pharmaceutical form
Film-coated tablets.
- Azmarda 50 mg is violet white ovaloid biconvex film-coated tablet with beveled edges, unscored, debossed with “NVR” on one side and “LZ” on the other side.
- Azmarda 100 mg is pale yellow ovaloid biconvex film-coated tablet with beveled edges, unscored, debossed with “NVR” on one side and “L1” on the other side.
- Azmarda 200 mg is light pink ovaloid biconvex film-coated tablet with beveled edges, unscored, debossed with “NVR” on one side and “L11” on the other side.
Active substances
Sacubitril/ Valsartan
Azmarda contains a salt complex of the anionic forms of sacubitril and valsartan, sodium cations, and water molecules in the molar ratio of 1:1:3:2.5 respectively. Following oral administration, Azmarda dissociates into sacubitril (which is further metabolized to LBQ657 [sacubitril]) and valsartan.
- Azmarda 50 mg film coated tablets contain (Sacubitril Valsartan).
- Azmarda 100 mg film coated tablets contain (Sacubitril Valsartan).
- Azmarda 200 mg film coated tablets contain (Sacubitril Valsartan).
Excipients
microcrystalline cellulose, low-substituted hydroxypropylcellulose, crospovidone, magnesium stearate (vegetable origin), talc, and colloidal silicon dioxide
Excipients of film coating:
hypromellose, titanium dioxide (E 171), Macrogol 4000, talc, iron oxide red (E 172)
For 50 and 200 mg: iron oxide black (E 172). For 100mg: iron oxide yellow (E 172).
- 5. What are the dosage and administration of Azmarda (Sacubitril Valsartan)?
Azmarda 200 mg twice daily is the target dose.
Azmarda 100 mg twice daily is the recommended starting dose.
Azmarda 50 mg twice daily as a starting dose is recommended for patients not currently taking an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB), and should be considered for patients previously taking low doses of these agents.
The dose of should be doubled every 2-4 weeks to achieve the target dose of Azmarda 200 mg twice daily, as tolerated by the patient.
Due to the potential risk of angioedema when used concomitantly with an ACE inhibitor, Azmarda must not be started until 36 hours after discontinuing ACE inhibitor therapy.
Azmarda should not be co-administered with an ARB due to the angiotensin II receptor blocking the activity of Azmarda.
If patients experience tolerability issues (symptomatic hypotension, hyperkalemia, renal dysfunction), consideration should be given to adjustment of concomitant medications, or to temporary down–titration of Azmarda. - 6. What is the clinical pharmacodynamics of Azmarda (Sacubitril Valsartan)?
The pharmacodynamic effects of Azmarda containing Sacubitril/ Valsartan were evaluated after single and multiple dose administrations in healthy subjects and in patients with heart failure, and are consistent with simultaneous neprilysin inhibition and RAAS blockade. In a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of Azmarda resulted in a significant non-sustained increase in natriuresis, increased urine cGMP, and decreased plasma MR-proANP and NT-proBNP compared to valsartan. In a 21-day study in HFrEF patients, Azmarda significantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1 compared to baseline. Azmarda also blocked the AT1-receptor as evidenced by increased plasma renin activity and plasma renin concentrations. In PARADIGM-HF, Azmarda decreased plasma NT-proBNP and increased plasma BNP and urine cGMP compared with enalapril. While BNP is a neprilysin substrate, NT-proBNP is not. Therefore, NT-proBNP (but not BNP) is a suitable biomarker for monitoring of heart failure patients treated with Azmarda.
In a thorough QTc clinical study in healthy male subjects, single doses of 400 mg and 1200 mg Azmarda had no effect on cardiac repolarization.
Neprilysin is one of multiple enzymes involved in the clearance of amyloid- beta (A-beta) from the brain and cerebrospinal fluid (CSF). Administration of Azmarda 400 mg once daily for 2 weeks to healthy subjects was associated with an increase in CSF A-beta 1-38 compared to placebo; there were no changes in concentrations of CSF A-beta 1-40 and 1-42. The clinical relevance of this finding is unknown.
- 7. What are the clinical pharmacokinetics of Azmarda (Sacubitril Valsartan)?
Absorption
Following oral administration, Azmarda dissociates into sacubitril, which is further metabolized to sacubitrilat, and valsartan, which reach peak plasma concentrations in 0.5 hours, 2 hours, and 1.5 hours, respectively. The oral absolute bioavailability of sacubitril and valsartan is estimated to be ≥ 60% and 23%, respectively. The valsartan in Azmarda is more bioavailable than the valsartan in other marketed tablet formulations.
Following twice daily dosing of Azmarda, steady-state levels of sacubitril, sacubitrilat, and valsartan are reached in 3 days. At steady state, sacubitril and valsartan do not accumulate significantly, while sacubitril accumulates by 1.6-fold. Azmarda administration with food has no clinically significant impact on the systemic exposures of sacubitril, sacubitril and valsartan. Although there is a decrease in exposure to valsartan when Azmarda is administered with food, this decrease is not accompanied by a clinically significant reduction in the therapeutic effect. Azmarda can therefore be administered with or without food.
Distribution
Azmarda is highly bound to plasma proteins (94% - 97%). Based on the comparison of plasma and CSF exposures, sacubitril does cross the blood brain barrier to a limited extent (0.28%). Azmarda has an apparent volume of distribution ranging from 75L to 103 L.
Biotransformation/metabolism
Sacubitril is readily converted to sacubitril by esterases; sacubitril is not further metabolized to a significant extent. Valsartan is minimally metabolized, as only about 20% of the dose is recovered as metabolites. A hydroxyl metabolite has been identified in plasma at low concentrations (<10%). Since CYP450 enzyme mediated metabolism of sacubitril and valsartan is minimal, co-administration with drugs that impact CYP450 enzymes is not expected to impact the pharmacokinetics.
Elimination
Following oral administration, 52 to 68% of sacubitril (primarily as sacubitril) and ~13% of valsartan and its metabolites are excreted in urine; 37 to 48% of sacubitril (primarily as sacubitril), and 86% of valsartan and its metabolites are excreted in feces.
Sacubitril, and valsartan are eliminated from plasma with a mean elimination half-life (T1/2) of approximately 1.43 hours, 11.48 hours, and 9.90 hours, respectively.
Linearity/non-linearity
The pharmacokinetics of sacubitril, sacubitril, and valsartan are linear in the dose range tested (Azmarda 50 to 400 mg).
- 8. What benefits of Azmarda (Sacubitril Valsartan) have been shown in studies?
There are multiples of clinical studies that established the benefits of Azmarda containing Sacubitril/ Valsartan in Heart Failure patients. Noteworthy highlights from major studies are as follows:
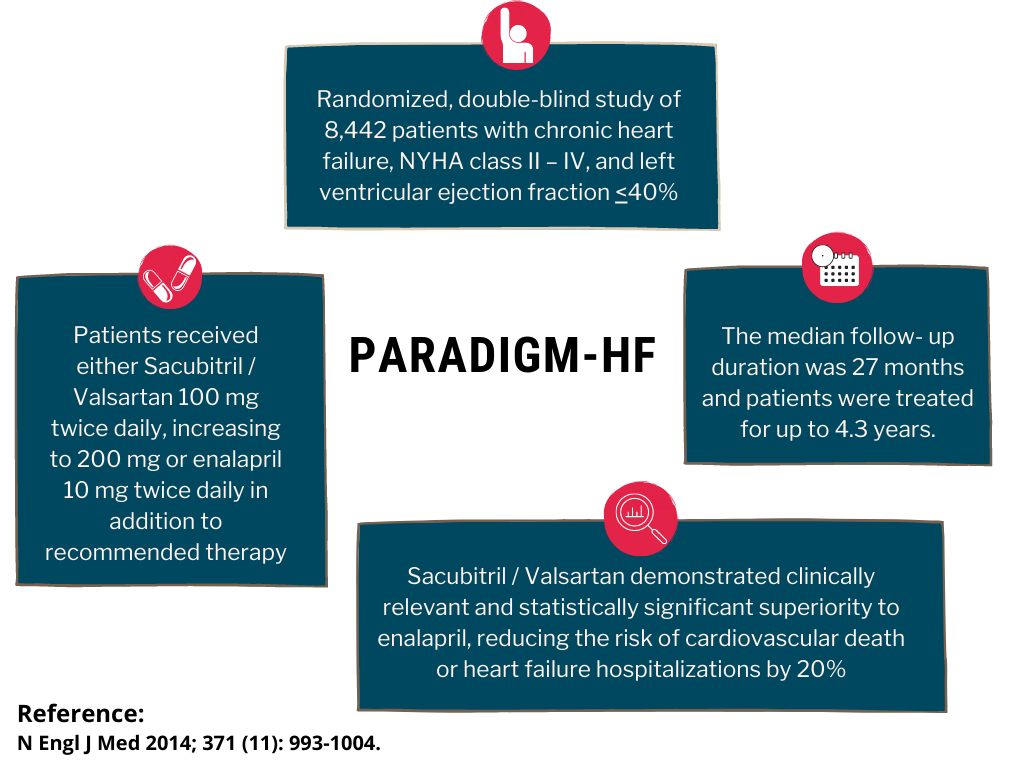
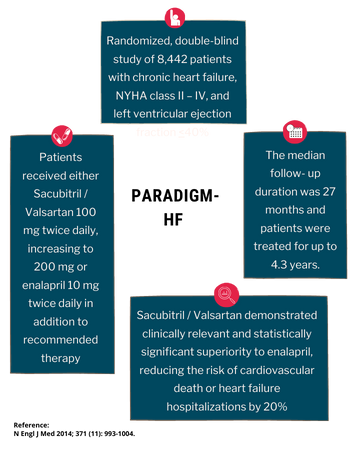
PARADIGM-HF
PARADIGM-HF was a multinational, randomized, double-blind study of 8,442 patients comparing Azmarda to enalapril, both given to adult patients with chronic heart failure, NYHA class II – IV, and systolic dysfunction (left ventricular ejection fraction ≤ 40%), in addition to other heart failure therapy. The primary endpoint was the composite of cardiovascular (CV) death or hospitalization for heart failure (HF).
Patients were required to discontinue their existing ACE inhibitor or ARB therapy and entered a sequential single-blind run-in period during which patients received treatment with enalapril 10 mg twice daily, followed by treatment with Azmarda 100 mg twice daily, increasing to 200 mg twice daily. Patients were then randomized to the double-blind period of the study to receive either Azmarda 200 mg or enalapril 10 mg twice daily [Azmarda (n= 4,209); enalapril (n= 4,233)].
Azmarda demonstrated clinically relevant and statistically significant superiority to enalapril, reducing the risk of cardiovascular death or heart failure hospitalizations by 20% (hazard ratio (HR): 0.80, 95% CI [0.73; 0.87], 1-sided p =0.0000002) versus enalapril.
A statistically significant reduction for CV death and first HF hospitalization was observed (CV death, RRR 20%, HR 0.80; 95% CI [0.71, 0.89], 1-sided p= 0.00004; and hospitalization for heart failure RRR 21%; HR 0.79; 95% CI 0.71, 0.89], 1-sided p= 0.00004).
Sudden death accounted for 45% of cardiovascular deaths and was reduced by 20% in Azmarda treated patients compared to enalapril treated patients (HR 0.80, p= 0.0082).
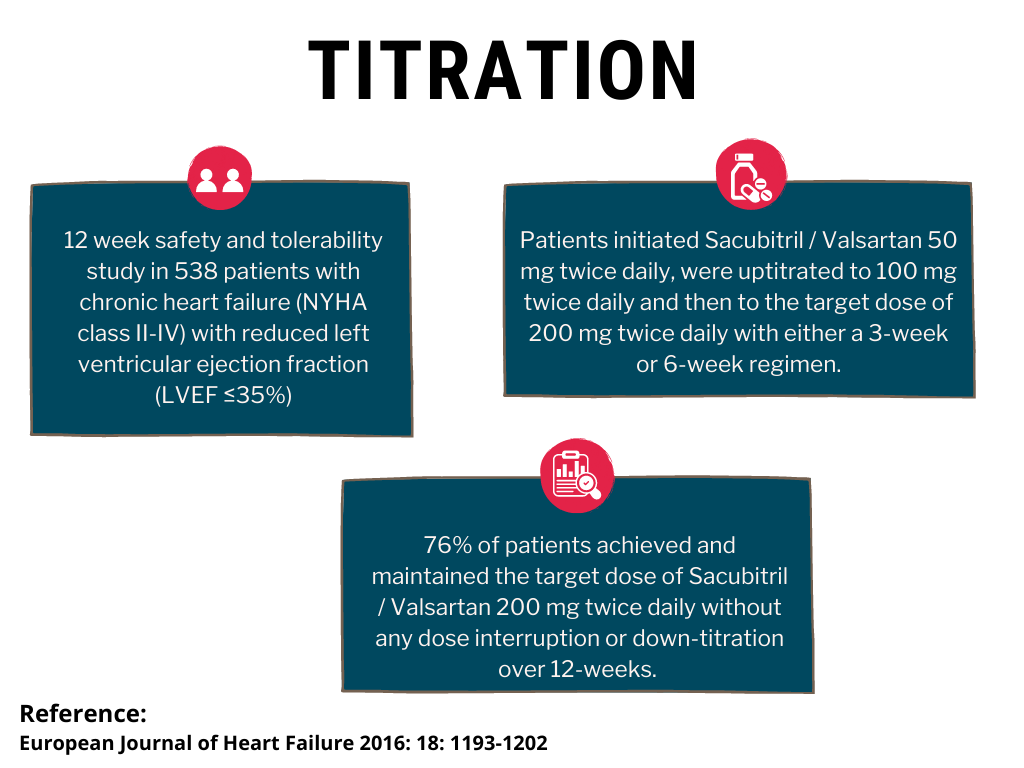
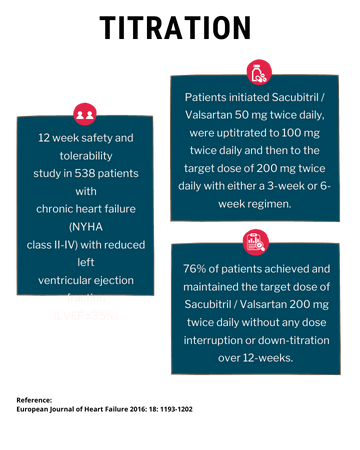
TITRATION
TITRATION was a 12-week safety and tolerability study in 538 patients with chronic heart failure (NYHA class II – IV) and systolic dysfunction (left ventricular ejection fraction ≤ 35%) naive to ACE inhibitor or ARB therapy or on varying doses of ACE inhibitors or ARBs prior to study entry. Patients initiated Azmarda 50 mg twice daily, were uptitrated to 100 mg twice daily and then to the target dose of 200 mg twice daily with either a 3-week or 6-week regimen.
Overall, 76% of patients achieved and maintained the target dose of Azmarda 200 mg twice daily without any dose interruption or down-titration over 12-weeks. More patients who were naïve to previous ACE inhibitor or ARB therapy or on low dose therapy (equivalent to < 10 mg of enalapril/ day) were able to achieve and maintain Azmarda 200 mg when uptitrated over 6 weeks versus 3 weeks.
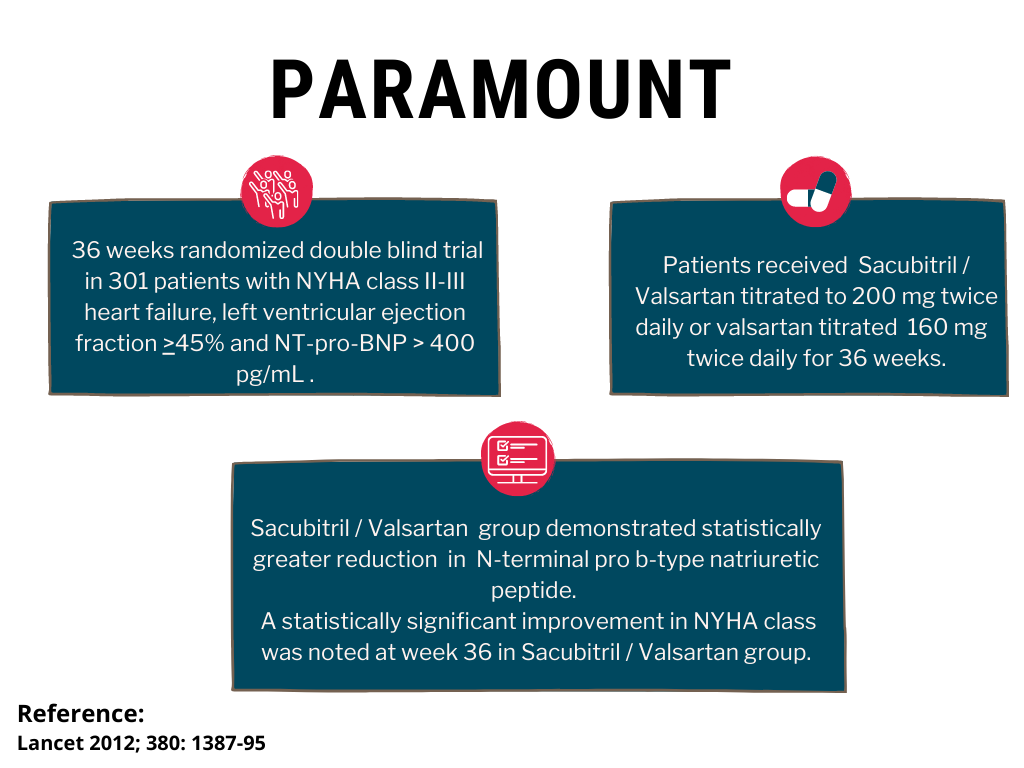
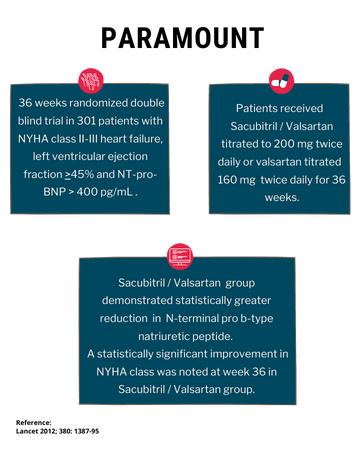
PARAMOUNT
PARAMOUNT, a randomized, double-blind trial in patients with left ventricular ejection fraction ≥ 45% comparing 200 mg of Azmarda (n=149) to 160 mg of valsartan (n=152) twice daily, demonstrated statistically greater reduction (p= 0.0050) in NT pro-BNP from baseline to week 12. The reduction from baseline in NT-proBNP was similar at weeks 12 and 36 in patients treated with Azmarda, while NT-proBNP decreased from week 12 to 36 in patients treated with valsartan. Significant reductions in left atrial size, both left atrial volume index (p=0.0069) and left atrial dimension (p=0.0337) were observed at week 36. A statistically significant improvement in NYHA class was noted at week 36 (p=0.0488).
- 9. What are the risks associated with Azmarda (Sacubitril Valsartan)? What are the precautions to be taken while using Azmarda (Sacubitril Valsartan)?
Fetal Toxicity
Azmarda can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. When pregnancy is detected, consider alternative drug treatment and discontinue Azmarda.
Angioedema
Azmarda may cause angioedema. In the double-blind period of PARADIGM-HF, 0.5% of patients treated with Sacubitril/Valsartan and 0.2% of patients treated with enalapril had angioedema. If angioedema occurs, discontinue Azmarda immediately, provide appropriate therapy, and monitor for airway compromise. Azmarda must not be re-administered.
Patients with a prior history of angioedema may be at increased risk of angioedema with Azmarda. Azmarda must not be used in patients with a known history of angioedema related to previous ACE inhibitor or ARB therapy. Azmarda should not be used in patients with hereditary angioedema.
Hypotension
Azmarda lowers blood pressure and may cause symptomatic hypotension. Patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), are at greater risk.
In the double-blind period of PARADIGM-HF, 18% of patients treated with Sacubitril/Valsartan and 12% of patients treated with enalapril reported hypotension as an adverse event, with hypotension reported as a serious adverse event in approximately 1.5% of patients in both treatment arms.
If hypotension occurs, consider dose adjustment of diuretics, concomitant antihypertensive drugs, and treatment of other causes of hypotension (e.g., hypovolemia). If hypotension persists despite such measures, reduce the dosage or temporarily discontinue Azmarda. Permanent discontinuation of therapy is usually not required.
Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system (RAAS), decreases in renal function may be anticipated in susceptible individuals treated with Azmarda.
In the double-blind period of PARADIGM-HF, 5% of patients in both the Sacubitril/ Valsartan and enalapril groups reported renal failure as an adverse event.
In patients whose renal function depends upon the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists has been associated with oliguria, progressive azotemia and, rarely, acute renal failure and death. Closely monitor serum creatinine, and down-titrate or interrupt Azmarda in patients who develop a clinically significant decrease in renal function.
Hyperkalemia
Through its actions on the RAAS, hyperkalemia may occur with Azmarda.
In the double-blind period of PARADIGM-HF, 12% of patients treated with Sacubitril/ Valsartan and 14% of patients treated with enalapril reported hyperkalemia as an adverse event/p>
Monitor serum potassium periodically and treat appropriately, especially in patients with risk factors for hyperkalemia such as severe renal impairment, diabetes, hypoaldosteronism, or a high potassium diet. Dosage reduction or interruption of Azmarda may be required.
- 10. What are the contraindications of Azmarda (Sacubitril Valsartan)? Azmarda is contraindicated with:
- Hypersensitivity to the active substance, sacubitril, valsartan, or any of the excipients.
- Concomitant use with ACE inhibitors. Azmarda must not be administered until 36 hours after discontinuing ACE inhibitor therapy.
- Known history of angioedema related to previous ACE inhibitor or ARB therapy.
- Hereditary angioedema
- Concomitant use with aliskiren in patients with Type 2 diabetes.
- Pregnancy
- 11. What are the adverse reactions of Azmarda (Sacubitril Valsartan)?
Clinically significant adverse reactions observed with Azmarda include:
- Hyperkalemia
- Hypotension
- Renal Impairment
- Renal Failure
- Diarrhea
- 12. What are the precautions to be taken for special populations groups while using Azmarda?
Renal impairment
A starting dose of 50 mg twice daily is recommended in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). Caution is recommended when using Azmarda in these patients due to limited data. No dose adjustment is required in patients with mild (eGFR 60-90 mL/min/1.73 m2) to moderate (eGFR 30-60 mL/min/1.73 m2) renal impairment.
Hepatic impairment
A starting dose of 50 mg twice daily is recommended for patients with moderate hepatic impairment (Child-Pugh B classification).
No dose adjustment is required when administering Azmarda to patients with mild hepatic impairment (Child-Pugh A classification).
No studies have been conducted in patients with severe hepatic impairment (Child-Pugh C classification). Therefore, use of Azmarda in these patients is not recommended.
Pediatric patients
The safety and efficacy of Azmarda in pediatric patients aged below 18 years have not been established.
Geriatric patients (older than 65 years)
No dosage adjustment is required in patients over 65 years.
- 13. What is the safety profile of Azmarda (Sacubitril Valsartan) in females of childbearing potential, pregnancy, breastfeeding, and fertility?
Females of childbearing potential (and contraceptive measures if applicable)
Female patients of childbearing potential should be advised about the consequences of exposure to Azmarda during pregnancy and to use contraception during treatment with Azmarda and for 1 week after their last dose.
Pregnancy
As for other drugs that also act directly on the RAAS, Azmarda must not be used during pregnancy. Azmarda exerts its effects via angiotensin II antagonism. As a result, a risk to the fetus cannot be excluded. There have been reports of injury to the developing fetus (e.g. spontaneous abortion, oligohydramnios and newborn renal dysfunction), when pregnant women have taken valsartan. Patients should be advised to discontinue Azmarda as soon as pregnancies occur and to inform their physicians.
Breast-feeding
It is not known whether Azmarda is excreted in human milk. The components of Azmarda, sacubitril and valsartan, were excreted in the milk of lactating rats. Because of the potential risk for adverse drug reactions in breastfed newborns/infants, Azmarda is not recommended during breastfeeding. A decision should be made whether to abstain from breast-feeding or to discontinue Azmarda while breast-feeding, taking into account the importance of Azmarda to the mother.
Fertility
There are no available data on the effect of Azmarda on human fertility. No impairment of fertility was demonstrated in studies with Azmarda in male and female rats.
- 14. What happens to the overdosage of Azmarda (Sacubitril Valsartan)?
Limited data are available with regard to overdosage in human subjects with Azmarda containing Sacubitril/ Valsartan. In healthy volunteers, a single dose of Azmarda 1200 mg, and 900 mg multiple doses (14 days) have been studied and were well tolerated.
Hypotension is the most likely symptom of overdosage due to the blood pressure-lowering effects of Azmarda. Symptomatic treatment should be provided.
Azmarda containing Sacubitril/ Valsartan is unlikely to be removed by hemodialysis due to high protein binding.
- 15. What measures are being taken to ensure the safe and effective use of Azmarda (Sacubitril Valsartan)?
Safety information has been included in the summary of product characteristics and the package leaflet for Azmarda, including the appropriate precautions to be followed by healthcare professionals and patients.