- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Aurobindo Pharma gets USFDA nod for generic osteoporosis drug
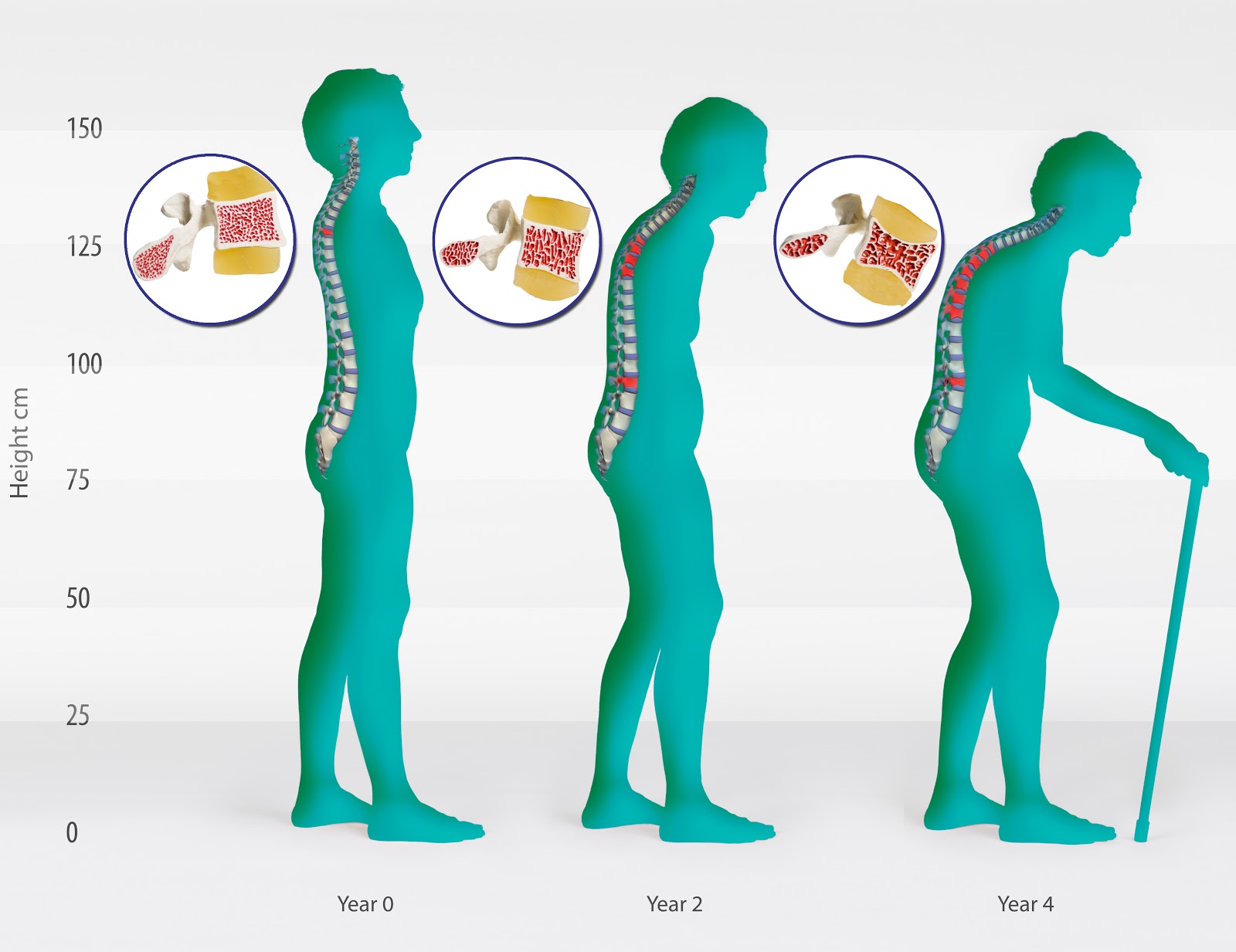
New Delhi, Aug 29 : Aurobindo Pharma today said it has received approval from the US FDA to manufacture and market its generic version of Eli Lilly's Evista tablets used to treat and prevent osteoporosis in postmenopausal women.
The approval by the USFDA for Raloxifene Hydrochloride tablets is for strength of 60 mg, which is bio and therapeutically equivalent to the reference listed drug product (RLD) Evista, 60 mg of Eli Lilly, the company said in a statement.
The product has an estimated market size of USD 404 million for the 12 months ended June 2015, the company added, citing IMS data.
This is the 45th Abbreviated New Drug Application (ANDA) to be approved out of Unit VII formulation facility in Hyderabad for manufacturing oral non-antibiotic products.
Aurobindo Pharma now has a total of 210 ANDA approvals, 182 final ones, including 9 from Aurolife Pharma LLC and 28 tentative approvals from USFDA, it added.
The approval by the USFDA for Raloxifene Hydrochloride tablets is for strength of 60 mg, which is bio and therapeutically equivalent to the reference listed drug product (RLD) Evista, 60 mg of Eli Lilly, the company said in a statement.
The product has an estimated market size of USD 404 million for the 12 months ended June 2015, the company added, citing IMS data.
This is the 45th Abbreviated New Drug Application (ANDA) to be approved out of Unit VII formulation facility in Hyderabad for manufacturing oral non-antibiotic products.
Aurobindo Pharma now has a total of 210 ANDA approvals, 182 final ones, including 9 from Aurolife Pharma LLC and 28 tentative approvals from USFDA, it added.
Meghna A Singhania is the founder and Editor-in-Chief at Medical Dialogues. An Economics graduate from Delhi University and a post graduate from London School of Economics and Political Science, her key research interest lies in health economics, and policy making in health and medical sector in the country. She is a member of the Association of Healthcare Journalists. She can be contacted at meghna@medicaldialogues.in. Contact no. 011-43720751
Next Story


