- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Breakthrough in Cardiac Care: Leadless Pacemaker-ICD System Exceeds Safety and Performance Goals, Study Reveals
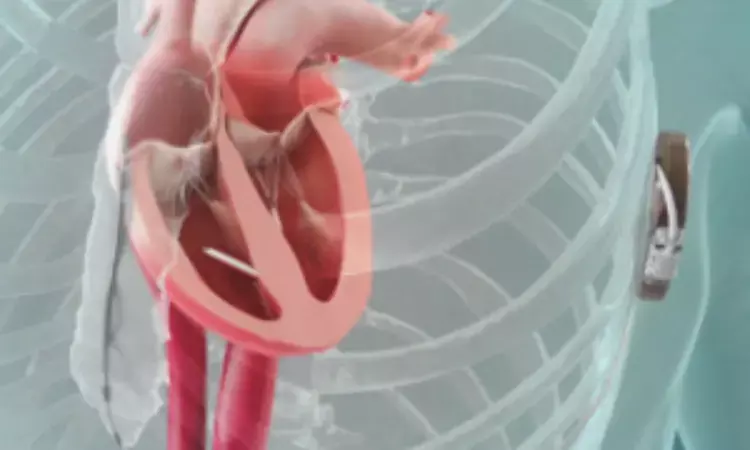
Netherlands: A recent prospective study demonstrated that the use of a leadless pacemaker capable of wireless communication with a subcutaneous implantable cardioverter-defibrillator (ICD) was free of pacemaker-related major complications. Additionally, the study confirmed successful communication between the devices and stable pacing thresholds maintained for up to six months.
The findings were published online in the New England Journal of Medicine.
The subcutaneous implantable cardioverter-defibrillator (ICD) is known to have fewer lead-related complications compared to a transvenous ICD but cannot provide bradycardia and antitachycardia pacing. To address this limitation, Reinoud E. Knops, Department of Clinical and Experimental Cardiology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands, and colleagues aimed to evaluate the safety of a modular pacing-defibrillator system combining a leadless pacemaker with a subcutaneous ICD, enabling wireless communication to deliver these pacing functions.
For this purpose, the researchers conducted a multinational, single-group study enrolling patients at risk for sudden death due to ventricular arrhythmias. Participants were monitored for six months following the implantation of a modular pacemaker-defibrillator system.
The safety endpoint was defined as freedom from major complications related to the leadless pacemaker, with a performance goal of 86%. The two primary performance endpoints were the successful communication between the pacemaker and the ICD, set against a performance goal of 88%, and maintaining a pacing threshold of up to 2.0 V at a 0.4-msec pulse width, with a performance goal of 80%.
The key findings of the study were as follows:
- A total of 293 patients were enrolled, 162 were included in the 6-month endpoint cohort and 151 completed the 6-month follow-up.
- The mean age of the participants was 60 years, 16.7% were women, and the average left ventricular ejection fraction was 33.1±12.6%.
- Freedom from a leadless pacemaker–related major complications was achieved in 97.5% of patients, surpassing the prespecified performance goal.
- Wireless communication between the pacemaker and ICD was successful in 98.8% of tests, exceeding the prespecified target.
- Of 151 patients, 97.4% had pacing thresholds of 2.0 V or less, surpassing the performance goal.
- Antitachycardia pacing successfully terminated 61.3% of arrhythmia episodes, with no failures due to communication issues.
- Out of 162 patients, 4.9% died during the study, but none of the deaths were related to arrhythmias or the implantation procedure.
"The leadless pacemaker, wirelessly communicating with a subcutaneous ICD, surpassed performance targets for major complication-free outcomes, successful device communication, and the proportion of patients achieving a pacing threshold of up to 2.0 V at a 0.4-msec pulse width over 6 months," the researchers concluded.
Reference: Knops RE, Lloyd MS, Roberts PR, Wright DJ, Boersma LVA, Doshi R, Friedman PA, Neuzil P, Blomström-Lundqvist C, Bongiorni MG, Burke MC, Gras D, Kutalek SP, Amin AK, Fu EY, Epstein LM, Tolosana JM, Callahan TD, Aasbo JD, Augostini R, Manyam H, Nair DG, Mondésert B, Su WW, Pepper C, Miller MA, Grammes J, Saleh K, Marquie C, Merchant FM, Cha YM, Cunnington C, Frankel DS, West J, Matznick E, Swackhamer B, Brisben AJ, Weinstock J, Stein KM, Reddy VY, Mont L; MODULAR ATP Investigators. A Modular Communicative Leadless Pacing-Defibrillator System. N Engl J Med. 2024 Oct 17;391(15):1402-1412. doi: 10.1056/NEJMoa2401807. Epub 2024 May 18. PMID: 38767244.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


