- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Edoxaban Matches Warfarin After Bioprosthetic Valve Surgery: ENBALV Trial Results
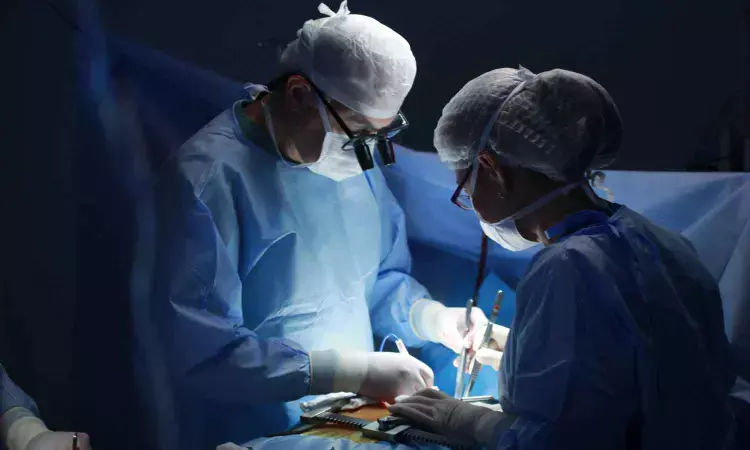
Edoxaban, an oral anticoagulant, was equally or more effective than warfarin in reducing the risk of stroke and blood clots for patients after heart valve replacement surgery, according to preliminary late-breaking science presented today at the American Heart Association’s Scientific Sessions 2024
. The meeting, Nov. 16-18, 2024, in Chicago, is a premier global exchange of the latest scientific advancements, research and evidence-based clinical practice updates in cardiovascular science.
Patients who receive a heart valve replacement are well-known to be at a high risk for stroke, blood clots and deep vein thrombosis, especially during the weeks immediately after surgery. Current treatment guidelines recommend anticoagulant therapy with medication to prevent and treat blood clots.
People who receive a mechanical heart valve require lifelong anticoagulant therapy to prevent thrombus (blood clot) formation on the valve. Patients who receive a bioprosthetic (made with human or animal tissue) heart valve typically need anticoagulants for three to six months after surgery.
The standard medication for anticoagulation in these patients is warfarin, which decreases the body’s ability to form blood clots by blocking the formation of clotting factors dependent on vitamin K.
“Currently, we can use only vitamin K antagonists such as warfarin for patients early after bioprosthetic valve surgery,” said lead study author Chisato Izumi, M.D., Ph.D., of the National Cerebral and Cardiovascular Center in Suita, Japan.
“However, warfarin has a narrow therapeutic range requiring frequent blood testing to monitor clotting activity, and it also interacts with other medications and food, which can be a challenge for patients and the health care professionals treating them.”
The ENBALV trial evaluated the effectiveness and safety of edoxaban, an oral anticoagulant that was approved by the FDA in 2015 for patients with non-valvular atrial fibrillation, compared to warfarin within three months after bioprosthetic valve surgery. Edoxaban works by blocking factor Xa, a clotting factor that plays a key role in the coagulation (blood clotting) process. Edoxaban is taken in a fixed dose and has a predictable pharmacokinetic profile, which means that its effects are not impacted by diet or other medications.
The randomized, multi-center trial included approximately 400 adults in Japan who had bioprosthetic heart valve replacement surgery at the aortic and/or mitral position. Researchers randomized the participants into two equal groups to receive either edoxaban or warfarin for 12 weeks after surgery.
The analysis found:
Edoxaban was equally or more effective than warfarin at preventing stroke and blood clots: 0.5% of patients receiving edoxaban had a stroke or systemic embolism, compared to 1.5% of patients receiving warfarin.
Major bleeding occurred in 4.1% of the edoxaban group and in 1% of the warfarin group. However, no fatal bleeding or intracranial hemorrhage was observed in patients treated with edoxaban, whereas one fatal cerebral hemorrhage occurred in the warfarin group.
Intracardiac thrombus (a blood clot in the heart) did not occur in any of the patients in the edoxaban group, but did occur in 1% of patients in the warfarin group.
Patients who received edoxaban did experience higher instances of gastrointestinal bleeding compared to patients who received warfarin (2.1% vs. 0%, respectively).
“Our findings show that edoxaban could help prevent blood clots and stroke as effectively as warfarin, indicating it is a viable post-surgery treatment alternative to consider for patients who have received a bioprosthetic heart valve replacement,” Izumi said. “Edoxaban could make life easier for patients recovering from heart valve surgery. Since this medication does not require regular blood tests to monitor anticoagulation activity and can be taken in a fixed dose, without fears of interaction with food or other medications, it reduces the burden on patients and improves their quality of life, especially in those crucial first few months after surgery.”
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


