- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA panel backs ultrasound-based renal denervation system for uncontrolled hypertension
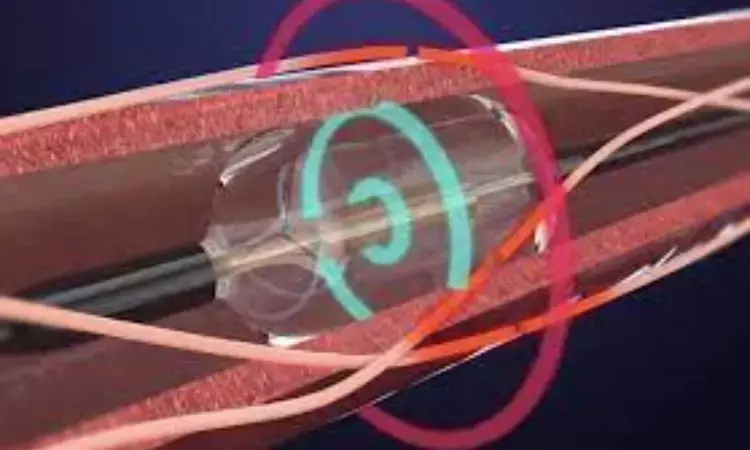
USA: An ultrasound renal denervation system has got backing from an FDA panel as a possible treatment for hypertension.
The FDA's Circulatory System Devices Panel voted 10-2 that the benefits of the Paradise ultrasound renal denervation system (ReCor) outweigh the risk for use in patients with uncontrolled hypertension intolerant or unresponsive to antihypertensive medications. The panel however expressed concerns about the long-term durability of the effect.
In the Medical Devices Advisory Committee Meeting, the panel members also voted 8-3, with one abstention, that ReCor's renal denervation system is effective for use in patients with uncontrolled hypertension, and voted unanimously (12-0) for the device's safety.
High blood pressure (BP) is a risk factor for stroke and heart disease affecting about 220 million people, only 12% have their BP under control with lifestyle interventions and available medications. The last new drug class for hypertension, the direct renin inhibitors, was approved in 2007, indicating the need for innovation.
The ultrasound renal denervation system is indicated to lower BP by ablation of the nerve surrounding the renal artery in adult patients with uncontrolled hypertension who may be inadequately responsive or intolerant to antihypertensive medications.
During the meeting, some panellists expressed concerns that renal denervation is not superior to antihypertensive medications and that while studies showed an acute reduction in BP with the procedure, the reduction was modest and there is no clarity on any long-term durability.
The Paradise system includes a catheter with an ultrasound transducer that is delivered percutaneously through the femoral artery to the renal artery under fluoroscopic guidance. An accompanying distal balloon contains cooling water to prevent arterial wall injury during thermal ablation.
"I voted 'yes' on efficacy with some misgivings, based on the heterogeneity of the response and small effect size," John Hirshfeld, Jr., MD, emeritus professor of medicine at the University of Pennsylvania Perelman School of Medicine, said after the vote. “I voted ‘yes’ because, since this is a novel mechanism for hypertension treatment [renal denervation] is an important tool to have in the toolbox. Hopefully, once in the toolbox, it will be used responsibly by the clinical community and promoted responsibly by the sponsor.”
The panel also debated the proposed indication for ultrasound renal denervation, stating that words such as “intolerant," “inadequately responsive" and “uncontrolled hypertension” were not clearly defined.
I voted ‘no’ for both the efficacy and the risk/safety profile because I did not feel comfortable voting ‘yes’ based on the precise wording of the indication. I voted yes for the safety," Benjamin Saville, PhD, director and senior statistical scientist at Berry Consultants in Austin, Texas, said after the vote.
“Based on all of the feedback from my clinical colleagues, the indication was my main concern. There is clearly a benefit in the study population ... that benefit demonstrated is more short-term. It is unknown what longer benefit we might have.”
Keith Allen, MD, director of surgical research at St. Luke’s Hospital of Kansas City, Missouri also expressed concerns about the durability of the effect with renal denervation. He voted 'no' on risk/benefit, despite this being a breakthrough device as he had real concerns based on its current listing and how it would be used in the real world.
Reference:
FDA. Circulatory System Devices Panel of the Medical Devices Advisory Committee (MDAC) Live Video. Presented Aug. 22, 2023. Accessed Aug. 22, 2023.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


