- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Leadless CRT system Using Ultrasound Energy Offers New Option for Heart Failure Management: SOLVE-CRT Study
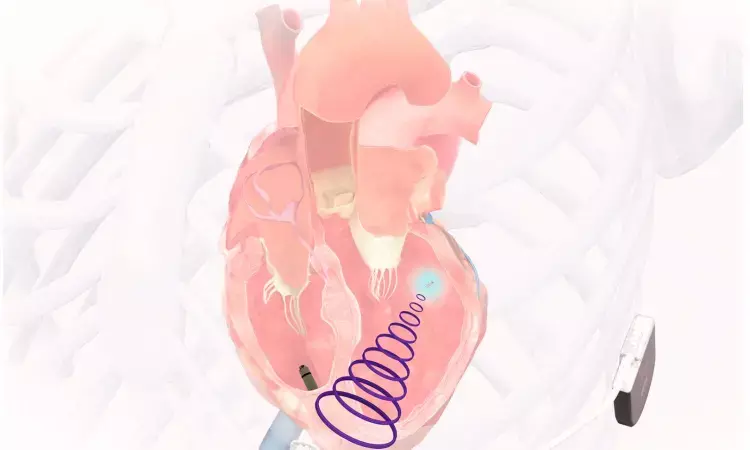
USA: A leadless left ventricular (LV) using ultrasound energy is promising as a viable alternative for patients with failed coronary sinus (CS) lead placement for cardiac resynchronization therapy (CRT), the SOLVE-CRT study has shown.
"In the prospective clinical trial involving 183 patients, 80.9% experienced no device- or procedure-related adverse events, while the average reduction in mean LV end-systolic volume was 16.4%," the researchers reported in JAMA Cardiology.
Heart failure (HF), a condition where the heart is unable to pump blood effectively, affects millions of people worldwide. For many patients, cardiac resynchronization therapy is a crucial treatment. Traditionally, CRT involves implanting a device with leads that stimulate the heart to improve its rhythm and function. However, this approach requires invasive procedures and carries risks associated with lead placement and device-related complications.
Previous studies have shown that about 40% of patients with HF who are eligible for CRT either fail to respond or are untreatable due to anatomical constraints. Considering this, Jagmeet P. Singh, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, and colleagues assess the efficacy and safety of a novel, leadless, LV endocardial pacing system for patients at high risk for a CRT upgrade or whose CS lead placement/pacing with a conventional CRT system failed.
The SOLVE-CRT study was a prospective multicenter trial conducted from 2018 through 2022, with follow-up data collected up to March 2023. It analyzed data from both an initial randomized, double-blind study involving 108 participants and a subsequent single-arm study with 75 participants. The trial spanned 36 centers across Australia, Europe, and the US and included patients who were nonresponders, previously untreatable (PU), or high-risk upgrades (HRU). All participants were included in the safety analysis, while the primary efficacy analysis focused on 100 patients, including 75 from the single-arm portion and 25 from the randomized treatment arm.
In the trial, patients were treated with the WiSE CRT System (EBR Systems), which featured a leadless LV endocardial pacing electrode stimulated using ultrasound energy delivered by a subcutaneously implanted transmitter and battery. The primary safety endpoint was the rate of freedom from type I complications, while the primary efficacy endpoint was the reduction in mean LV end-systolic volume (LVESV).
The study led to the following findings:
- The study included 183 participants; the mean age was 68.1 years, and 77% were males.
- The trial was terminated at an interim analysis for meeting prespecified stopping criteria.
- In the safety population, patients were either New York Heart Association Class II (34.6%) or III (65.4%).
- The primary efficacy endpoint was met with a 16.4% reduction in mean LVESV.
- The primary safety endpoint was met with an 80.9% rate of freedom from type I complications, which included 12 study device system events (6.6%), five vascular events (2.7%), three strokes (1.6%), and seven cardiac perforations which occurred early in the study (3.8%).
"The SOLVE-CRT study has shown that using the WiSE CRT system for leadless LV endocardial pacing leads to a reduction in LVESV in heart failure patients. This innovative system could offer an alternative to traditional CRT implants for certain populations with heart failure," the researchers concluded.
Reference:
Singh JP, Rinaldi CA, Sanders P, et al. Leadless Ultrasound-Based Cardiac Resynchronization System in Heart Failure. JAMA Cardiol. Published online July 31, 2024. doi:10.1001/jamacardio.2024.2050
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


