- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Novel percutaneous ventricular assist device beneficial in patients undergoing high-risk PCI
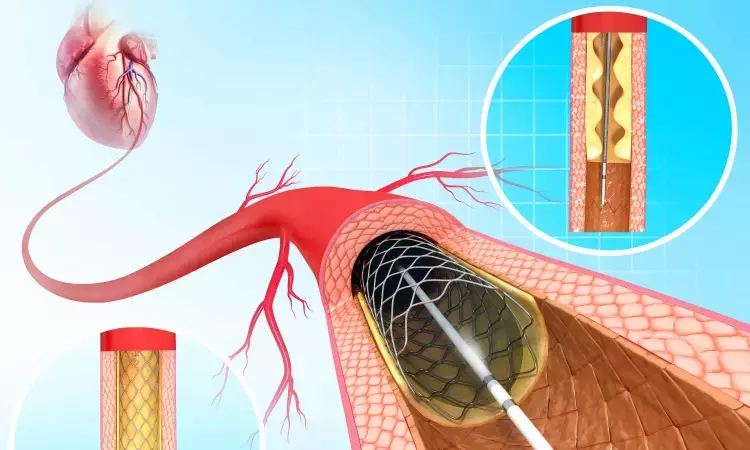
Findings from the first-in-human trial of a novel percutaneous ventricular assist device (pVAD) were presented today as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 Scientific Sessions. The initial results demonstrated that Supira Medical’s low profile, high continuous flow device allowed for successful procedure completion for patients undergoing high-risk percutaneous coronary interventions (PCI).
PCI is a non-surgical procedure that uses a catheter to place a stent and open blood vessels in the heart that have been narrowed by plaque buildup. Commonly used during these interventions in high-risk patients, pVADs are mechanical pumps that provide hemodynamic blood flow. Current FDA-approved pVAD devices typically have large profiles and are associated with high rates of morbidity and vascular complications such as vessel injury requiring surgical repair, loss of limb, hematoma, and/or acute limb ischemia.
Supira’s novel low profile system aims to support cardiovascular hemodynamics with minimal hemolysis and a low risk of vascular complications for patients undergoing high-risk PCI and those in cardiogenic shock, a condition in which the heart is suddenly and/or chronically damaged and cannot support flow into the vital organs.
This first-in-human (FIH) study aimed to demonstrate the safety and performance of Supira’s low profile, high continuous flow system for cardiovascular hemodynamic support during high-risk PCI. As part of this prospective, single-arm, single-center study, six patients underwent high-risk PCI with the Supira system. Primary endpoints included procedural and safety outcomes. Procedural success was defined as safe implantation of device, hemodynamic support during the PCI, and successful withdrawal of the device. Safety assessment was defined as freedom from device-related major adverse events (MAE), including vascular complications (access and central), aortic valve injury, mitral valve injury, and systemic embolization.
Procedural success was 100% and none of the patients treated with the Supira system experienced device-related adverse events peri-procedurally. Additionally, no early indicators of clinically significant hemolysis were reported. Patient were age 58±9 years, 83% male and a body mass index of 30±2. Comorbid conditions consisted of prior heart attack (66%), diabetes (50%), tobacco use (50%) and pulmonary hypertension (50%). The ejection fraction ranged from 30-60% with at least 2 vessels treated in 66% of the patients. The device support time ranged from 42-96 minutes.
“Initial safety results of the Supira’s pump performance are promising and point to a meaningful advancement which could allow more operators to confidently perform high-risk PCI procedures while avoiding vascular complications,” said Gagan Singh M.D., M.S., Director of Clinical Cardiovascular Research at University of California, Davis, presenting author and study lead. “This system features a unique combination of low profile and high continuous flow that offers the potential to minimize vascular complications and hemolysis while aiming to provide operators with a single device for multiple pVAD indications.”
Reference:
Novel percutaneous ventricular assist device feasibile in patients undergoing high-risk percutaneous coronary interventions (HRPCI), Society for Cardiovascular Angiography and Interventions, Meeting, SCAI Scientific Sessions.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


