- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
PULSED-AF: Pulsed-field ablation safe and effective for treating atrial fibrillation
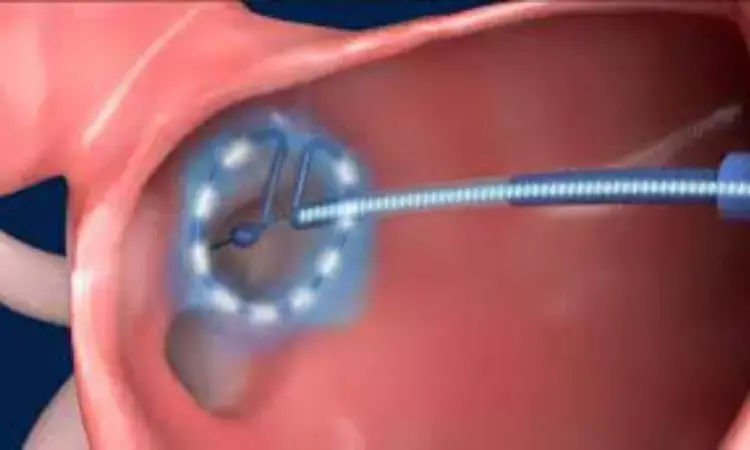
USA: Recent data from the prospective PULSED-AF study has shown that pulsed field ablation (PFA) for pulmonary vein isolation (PVI) safely and successfully abolished atrial arrhythmias over one year in 80% of the patients with atrial fibrillation (AF).
The 300-patient trial, the findings of which are published in Circulation, was designed for the US FDA (Food and Drug Administration) consumption as it considered approval of the tested device, Medtronic's PulseSelect PFA system.
The trial showed a low rate of primary safety adverse events (0.7%) and effectiveness consistent with established ablation technologies using novel irreversible electroporation energy for treating atrial fibrillation patients.
Radiofrequency catheter ablation in some forms of atrial fibrillation has been a standout success but not an unqualified success —it has safety issues, doesn't always work and has other limitations. Thus, the continued search for alternative arrhythmia ablation technologies has notably produced cryo-balloons and, lately, an exotic assortment of pulsed-field ablation catheters.
Pulse field ablation utilizes electrical pulses to cause nonthermal irreversible electroporation and produce cardiac cell death. Pulsed-field ablation may have effects comparable to traditional catheter ablation while preventing thermally mediated complications.
Atul Verma, McGill University Health Centre, Montreal, QC, Canada, and colleagues conducted the PULSED AF pivotal study, a prospective, global, multicenter, nonrandomized, paired single-arm study. It included patients with persistent (n=150) or paroxysmal (n=150) AF despite class I or III antiarrhythmic drugs at 41 centres in Western Europe, North America, Australia, and Japan treated with PFA. Using weekly and symptomatic trans telephonic monitoring consisting of 6- and 12-month 24-hour Holter monitoring and 3-, 6-, and 12-month ECGs, all patients were monitored for one year.
The primary safety endpoint was freedom from a composite of serious adverse events related to the procedure or device. Freedom from a composite of arrhythmia recurrence, acute procedural failure, or antiarrhythmic escalation through 12 months was determined, excluding a 3-month blanking period allowing recovery (primary effectiveness endpoint). Primary endpoints were evaluated using Kaplan-Meier methods.
The authors reported the following findings:
- Pulsed-field ablation was effective at one year in 66.2% of patients with paroxysmal AF and 55.1% with persistent AF.
- The primary safety endpoint occurred in 1 patient (0.7%) in the paroxysmal and persistent AF cohorts.
To conclude, PULSED AF showed a low rate of primary safety adverse events and provided effectiveness consistent with established ablation technologies using novel irreversible electroporation energy to treat AF patients.
Reference:
The study, "Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial" was published in Circulation. DOI: https://doi.org/10.1161/CIRCULATIONAHA.123.063988
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


