- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Researchers develop gel that safely whitens teeth when exposed to near infrared light
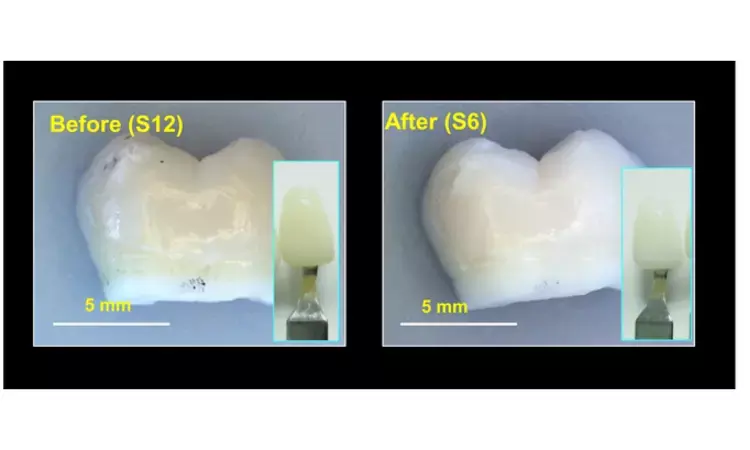
Most people would like to flash a smile of pearly whites, but over time teeth can become stained by foods, beverages and some medications. Unfortunately, the high levels of hydrogen peroxide in dentists' bleaching treatments can damage enamel and cause tooth sensitivity and gum irritation.
Now the researchers have developed a gel that, when exposed to near infrared (NIR) light, safely whitens teeth without the burn.
The study has been published in ACS Applied Materials & Interfaces.
The growing demand for selfie-ready smiles has made tooth whitening one of the most popular dental procedures. Treatments at a dentist's office are effective, but they use high-concentration hydrogen peroxide (30–40%). Home bleaching products contain less peroxide (6–12%), but they usually require weeks of treatment and don't work as well. When a bleaching gel is applied to teeth, hydrogen peroxide and peroxide-derived reactive oxygen species (mainly the hydroxyl radical) degrade pigments in stains. The hydroxyl radical is much better at doing this than hydrogen peroxide itself, so researchers have tried to improve the bleaching capacity of low-concentration hydrogen peroxide by boosting the generation of powerful hydroxyl radicals. Because previous approaches have had limitations, Xingyu Hu, Li Xie, Weidong Tian and colleagues wanted to develop a safe, effective whitening gel containing a catalyst that, when exposed to NIR light, would convert low levels of hydrogen peroxide into abundant hydroxyl radicals.
The researchers made oxygen-deficient titania nanoparticles that catalyzed hydroxyl radical production from hydrogen peroxide. Exposing the nanoparticles to NIR light increased their catalytic activity, allowing them to completely bleach tooth samples stained with orange dye, tea or red dye within 2 hours. Then, the researchers made a gel containing the nanoparticles, a carbomer gel and 12% hydrogen peroxide. They applied it to naturally stained tooth samples and treated them with NIR light for an hour. The gel bleached teeth just as well as a popular tooth whitening gel containing 40% hydrogen peroxide, with less damage to enamel. The nanoparticle system is highly promising for tooth bleaching and could also be extended to other biomedical applications, such as developing antibacterial materials, the researchers say.
The authors acknowledge funding from the National Natural Science Foundation of China, the National Key R&D Program of China and the Key Technologies R&D Program of Sichuan Province.
The abstract that accompanies this paper is available here.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS' mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world's scientific knowledge. ACS' main offices are in Washington, D.C., and Columbus, Ohio.
https://pubs.acs.org/doi/10.1021/acsami.1c06774
Hina Zahid Joined Medical Dialogue in 2017 with a passion to work as a Reporter. She coordinates with various national and international journals and association and covers all the stories related to Medical guidelines, Medical Journals, rare medical surgeries as well as all the updates in the medical field. Email: editorial@medicaldialogues.in. Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


