- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Abrocitinib proves efficacious in atopic dermatitis : JAMA study
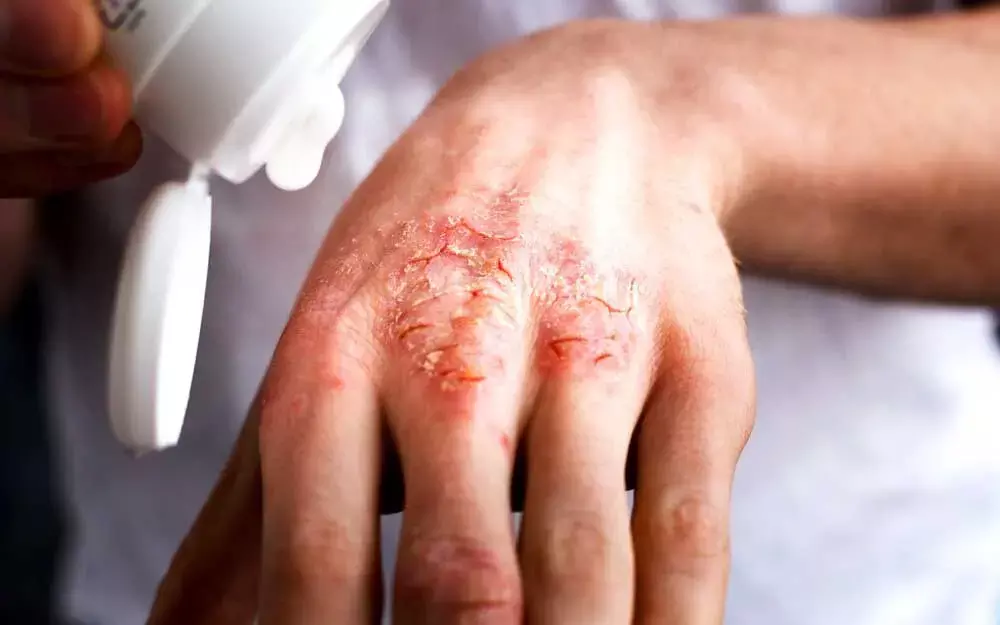
Abrocitinib proves efficacious in atopic dermatitis finds a new study published in JAMA. Atopic dermatitis (AD) is a severely pruritic chronic, relapsing inflammatory cutaneous disease. Dupilumab, a monoclonal antibody is one of the systemic drug approved for treating moderate-to-severe AD in adolescents but an efficacious and safe oral treatment for AD is an unmet need. Recently a study demonstrating efficacy of abrocitinib, a Janus kinase (JAK) 1–selective inhibitor in atopic dermatitis was published In JAMA Dermatology.
This was JADE TEEN study, a phase 3, randomized, double-blind, placebo-controlled parallel study conducted between February 18, 2019, and April 8, 2020 in patients with moderate-to-severe AD.
Patients were randomly assigned 1:1:1 to receive once-daily oral abrocitinib 200mg or 100mg, or placebo for 12 weeks in combination with topical therapy. Patients were screened within 28 days of the first dose followed by treatment with a study drug for 12 weeks.
Eligible patients characteristics at baseline -
1. aged 12 to 17 years
2. bodyweight of 55 lb or greater
3. confirmed diagnosis of AD according to the diagnostic criteria of Hanifin and Rajka
4. Investigator's Global Assessment [IGA] score of ≥3
5. Eczema Area and Severity Index [EASI]16 score of ≥16
6. Affected body surface area [BSA], ≥10%
7. Peak Pruritus Numerical Rating Scale [PP-NRS (used with permission of Regeneron Pharmaceuticals and Sanofi SA)] score of ≥4)
8.inadequate response to 4 consecutive weeks or longer of topical medication within 6 months before screening
9. candidates for systemic therapy
10. prior documentation of varicella-zoster virus immunity
IGA response of clear (0) or almost clear (1) with improvement of 2 or more grades from baseline (IGA 0/1)
75% or greater improvement from baseline in Eczema Area and Severity Index (EASI-75) response at week 12
Secondary end point- 4-point or greater improvement in Peak Pruritus Numerical Rating Scale (PP-NRS4) at week 12
Results
The study involved 285 adolescents with moderate-to-severe AD (145 males [50.9%] and 140 females [49.1%]), of whom 160 (56.1%) were White and 94 (33.0%) were Asian
Median age of patients was 15 years (interquartile range 13-17 years)
Significantly more patients treated with abrocitinib (200mg or 100mg) vs placebo achieved an IGA response of 0/1 (46.2%; 41.6%vs 24.5%; P < .05 for both)
At week 12, EASI-75 responses occurred in 67 (72.0%) and 61 (68.5%) patients in abrocitinib 200 mg and 100 mg respectively vs 39 (41.5%) patients in the placebo group which was significant when each group was individually compared to placebo group (P < .05)
The proportion of patients who achieved a PP-NRS4 response was significantly higher for the abrocitinib groups than placebo (55.4%; 52.6%vs 29.8%; P < .01) at week 12
Decreases from baseline in Pruritus and Symptoms Assessment for AD (PSAAD) scores were greater for each abrocitinib group vs placebo at all times assessed
For Scoring of AD index SCORAD- 50, SCORAD-75, and a SCORAD sleep loss visual analog scale score of less than 2, responses were higher for the abrocitinib groups than placebo
From weeks 2 to 12, adolescents reported improvement inQoL, with greater improvements in CDLQI scores for the abrocitinib groups than placebo
Adverse events were reported for 59 (62.8%), 54 (56.8%), and 50 (52.1%) patients in the 200mg, 100mg, and placebo groups, respectively; nausea was more common with abrocitinib, 200mg (17 [18.1%]) and 100mg (7 [7.4%]). Herpes-related AEs were infrequent; 1 (1.1%), 0, and 2 (2.1%) patients had serious AEs. There were modest increases in total cholesterol and high and low-density lipoprotein cholesterol levels for both abrocitinib doses vs placebo that plateaued at week 4. Dose-related decreases in median platelet cell counts were observed in patients who were treated with abrocitinib, with a nadir at week 4
To conclude this randomized clinical trial found oral abrocitinib 200mg or 100mg combined with topical corticosteroids was to be efficacious and well-tolerated in adolescents with moderate-to-severe AD, with a favourable safety profile.
Source
Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, Yao Z, Takahashi H, Barbarot S, Feeney C, Zhang F, DiBonaventura M, Rojo R, Valdez H, Chan G. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA Dermatol. 2021 Aug 18. doi: 10.1001/jamadermatol.2021.2830. Epub ahead of print. PMID: 34406366
MBBS
Dr Manoj Kumar Nayak has completed his M.B.B.S. from the prestigious institute Bangalore medical college and research institute, Bengaluru. He completed his M.D. Dermatology from AIIMS Rishikesh. He is actively involved in the field of dermatology with special interests in vitiligo, immunobullous disorders, psoriasis and procedural dermatology. His continued interest in academics and recent developments serves as an inspiration to work with medical dialogues.He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


