- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Belimumab improves cutaneous lupus erythematosus in patients with or without SLE
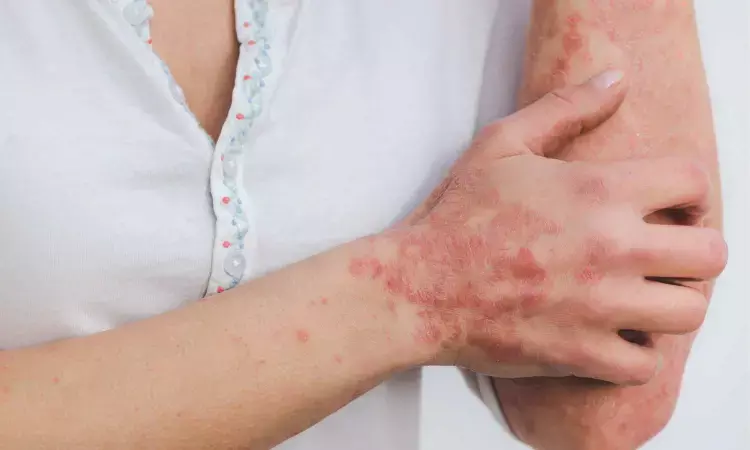
New research presented this week at ACR Convergence 2022, the American College of Rheumatology's annual meeting, found that the B-cell inhibitor belimumab significantly improved cutaneous lupus erythematosus (CLE) whether or not patients also had systemic lupus erythematosus (SLE or lupus)
CLE is an autoimmune disease primarily affecting the skin. Lesions develop on sun-exposed areas of the body and may be the sole manifestation of lupus or exist with systemic disease. In addition to psychological distress, CLE can also cause scarring and pigment changes – especially in darker skin. Belimumab, a monoclonal antibody that inhibits B-cell activation, is approved for SLE but less is known about its use in CLE, including the time to response after starting the drug.
Researchers undertook this meta-analysis to examine the efficacy of belimumab and the time to response in CLE patients with or without SLE.
After a comprehensive manual search of more than 700 interventional and observational studies, the researchers identified six blinded interventional studies to include in the meta-analysis, where belimumab was compared to the standard of care, such as conventional disease-modifying anti-rheumatic drugs (DMARDs) or prednisone plus hydroxychloroquine. Included studies were homogenous, with a low risk of bias.
The primary outcome was clinical response at 52 weeks in belimumab users compared to non-users. Clinical response was defined as a decrease in skin manifestations from a baseline British Isles Lupus Assessment Group (BILAG) score of A or B to a BILAG score of B-E. The BILAG index is a validated measure of disease activity in SLE.
The researchers calculated the pooled odds ratio for each consecutive four-week interval to find the clinical response time and the time to sustained response in belimumab users compared to nonusers. They also pooled the odds of skin flares at one year.
The resulting analysis showed the odds of clinical response at 52 weeks were 44% higher in patients using belimumab. Clinical response was first noted 20 weeks after staring the drug, with a sustained clinical response peaking at one year. Patients using belimumab also had a 49% lower risk of severe skin flares.
"Our study is the first meta-analysis of belimumab's efficacy in CLE and highlights that it is an effective adjunct therapy for patients with CLE as a primary manifestation of SLE," says Shivani Garg, MD, MS, Assistant Professor at the University of Wisconsin School of Medicine and Public Health and the study's lead author. "The study also highlights that belimumab can take at least 20 weeks to achieve a sustained clinical response in patients with CLE, so it should not be prematurely discontinued in these patients."
Dr. Garg also points to findings showing that belimumab users have a nearly 50% lower chance of a severe cutaneous flare compared to nonusers.
The study did not examine the comparative efficacy of belimumab in different subtypes of CLE, but Dr. Garg says it "lays a foundation for future studies across CLE subtypes."
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


