- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Donidalorsen Therapy Effective Against Hereditary Angioedema: NEJM
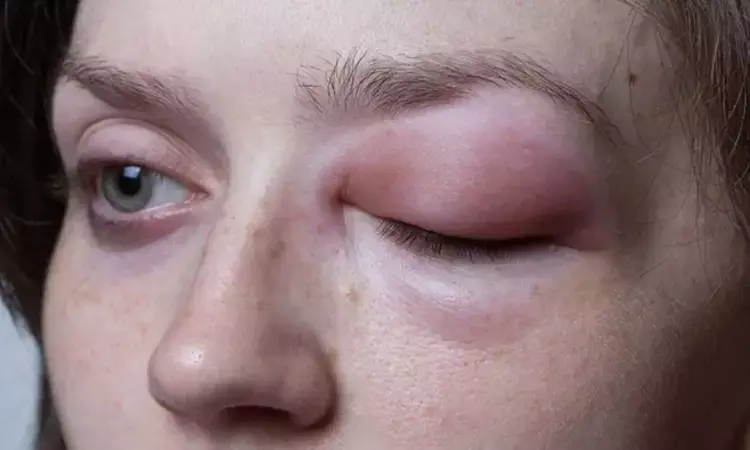
A recent phase published in the New England Journal of Medicine 3 trial demonstrated that donidalorsen, an antisense oligonucleotide, significantly reduces the frequency of hereditary angioedema (HAE) attacks. This rare disorder is characterized by episodic and potentially life-threatening swelling due to kallikrein–kinin dysregulation and may soon have a more effective long-term prophylactic option.
The double-blind, randomized trial inluded a total of 90 patients with hereditary angioedema who were assigned to receive either donidalorsen or a placebo. Donidalorsen was administered subcutaneously at a dose of 80 mg every 4 weeks (45 patients) or every 8 weeks (23 patients), while 22 patients received a placebo. The primary endpoint of the study was the time-normalized number of investigator-confirmed HAE attacks per 4 weeks, measured from week 1 to week 25.
The results of this trial found that patients receiving donidalorsen every 4 weeks underwent a least-squares mean attack rate of 0.44 attacks per 4 weeks, while the individuals on an 8-week schedule had a rate of 1.02 and the placebo group had an attack rate of 2.26. This translates to an 81% reduction in the attack rate for the 4-week group when compared to the placebo, with a median reduction of 90% from baseline. For the 8-week group, the reduction was 55% with a median decrease of 83%. During the period from weeks 5 to 25, the 4-week group saw an 87% reduction in attack rates when compared to placebo, while the 8-week group underwent a 60% reduction. These figures illuminate the potential of donidalorsen as a highly effective prophylactic treatment.
Despite reducing attack rates, donidalorsen also significantly improved the quality of life for patients. At week 25, the least-squares mean total score on the Angioedema Quality-of-Life Questionnaire improved by 18.6 points more in the 4-week group when compared to the placebo group. This improvement highlights the substantial impact donidalorsen can have on daily living for the individuals with HAE. Safety data from the trial indicated that donidalorsen was generally well-tolerated. The most common adverse events included erythema at the injection site, headache and nasopharyngitis with 98% of these events being mild or moderate in severity.
The findings from this phase 3 trial suggest that donidalorsen as a promising candidate for the long-term prophylactic treatment of hereditary angioedema. Donidalorsen could offer new hope for the individuals with HAE by significantly reducing attack rates and improving the quality of life of patients.
Reference:
Riedl, M. A., Tachdjian, R., Lumry, W. R., Craig, T., Karakaya, G., Gelincik, A., Stobiecki, M., Jacobs, J. S., Gokmen, N. M., Reshef, A., Gompels, M. M., Manning, M. E., Bordone, L., Newman, K. B., Treadwell, S., Wang, S., Yarlas, A., & Cohn, D. M. (2024). Efficacy and Safety of Donidalorsen for Hereditary Angioedema. In New England Journal of Medicine. Massachusetts Medical Society. https://doi.org/10.1056/nejmoa2402478
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


