- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Dupilumab Effective and Safe for Severe Atopic Dermatitis in Infants and Young Children: Study
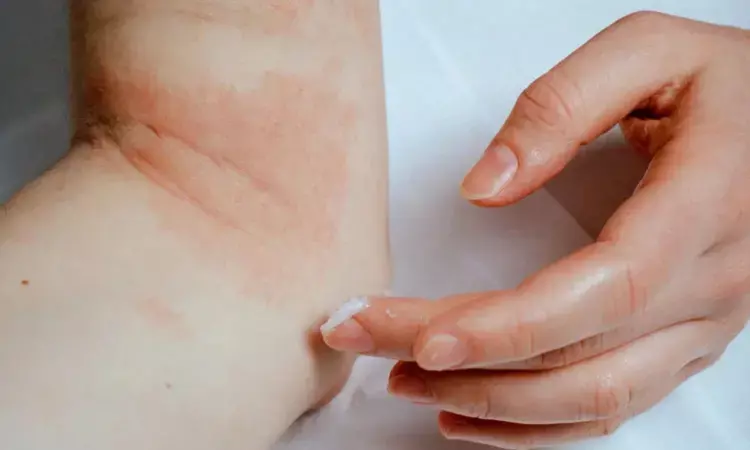
A new study published in the Journal of the European Academy of Dermatology & Venerology showed that in children with severe atopic dermatitis (AD) between the ages of 6 months and 5 years, dupilumab demonstrated notable effectiveness and a favorable safety profile, which is in line with results previously documented in older pediatric and adult populations.
A human monoclonal anti-interleukin-4/13 antibody called dupilumab is authorized for the treatment of severe atopic dermatitis in children. In October 2022, the French Health Authority approved it for an Early Access Program (EAP) for youngsters. This EAP assessed the safety and efficacy of dupilumab in children (6 months to 5 years old) with severe AD who need systemic therapy.
Analysis was done using cumulative data from the French EAP. Every 4 weeks, children with severe AD were given dupilumab 200 and 300 mg using a pre-filled syringe (children weighing ≥5 to <15 kg and ≥15 to <30 kg, respectively). At baseline and follow-up months (M) 1, 3, and 6, the Investigator's Global Assessment (IGA), Eczema Area and Severity Index (EASI) 50/75/90, Pruritus-Numerical Rating Scale (P-NRS), Infants' Dermatitis Quality of Life Index (IDQOL; for children under 4 years), and Children's Dermatology Life Quality Index (CDLQI; for children over 4 years. Safety was assessed.
A total of 198 children (<2 years old: N = 27 [13.6%]; ≥2 years old: N = 171 [86.4%]) were enrolled. 83.7% of the 116 assessed patients (exposed patients with at least one follow-up form) had an IGA score of 0/1, 94.4%/75.0%/44.4% had an EASI score of 50/75/90, and 67.6% had a P-NRS score improvement of at least 4 points, at M6.
The IGA, EASI, and P-NRS scores decreased by an average of −2.1 ± 1.2, −17.5 ± 11.6, and −4.4 ± 2.8, respectively, from the start of therapy to M6. From the start of therapy to M6, the mean ± SD IDQOL and CDLQI scores decreased numerically by −6.9 ± 6.6 and −8.8 ± 7.3, respectively. Safety was in line with dupilumab's recognized safety profile. Overall, the EAP's 6-month follow-up outcomes were in line with those seen in dupilumab randomized clinical trials including children with moderate-to-severe AD between the ages of 6 months and 5 years.
Source:
Lasek, A., Bellon, N., Mallet, S., Aubert, H., Vera, C., Achour, L., Thénié, C., Prescilla, R., Abasq-Thomas, C., & Barbarot, S. (2025). Dupilumab treatment in children aged 6 months to 5 years with severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology, jdv.70268. https://doi.org/10.1111/jdv.70268
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


