- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Evaluation of Biologic Efficacy and Cost per NNT in Moderate-to-Severe Psoriasis: Indian Analysis

Evaluation of Biologic Efficacy and Cost per NNT in Moderate-to-Severe Psoriasis: Indian Analysis. Ixekizumab and ustekinumab were the most cost-effective biologics to achieve Psoriasis Area and Severity Index (PASI)100 and PASI90 responses, based on a cost per number needed to treat (NNT) analysis based on MRP for moderate-to-severe plaque psoriasis (PsO), as reported by a recently...
Evaluation of Biologic Efficacy and Cost per NNT in Moderate-to-Severe Psoriasis: Indian Analysis. Ixekizumab and ustekinumab were the most cost-effective biologics to achieve Psoriasis Area and Severity Index (PASI)100 and PASI90 responses, based on a cost per number needed to treat (NNT) analysis based on MRP for moderate-to-severe plaque psoriasis (PsO), as reported by a recently published study.
The findings of the study were published in August 2025 in the Indian Journal of Dermatology, Venereology and Leprology, highlighting that the cost per NNT data generated in the analysis can assist procurement agencies and dermatologists in estimating the expected cost to patients, both in the short and long term bases in psoriasis.
Plaque psoriasis (PsO), the most common form of psoriasis, affects about 80% of patients and significantly impacts quality of life (QoL). The introduction of biologics has transformed the management of moderate-to-severe PsO, with newer agents achieving rapid clearance of skin lesions and high PASI 75/90/100 responses, along with notable improvements in Dermatology Life Quality Index (DLQI) compared with conventional therapies. Despite their superior efficacy, the high cost of biologics remains a major barrier for Indian dermatologists when prescribing treatment. A tool that compares treatment efficacy with cost could help support clinical decision-making.1
A study was conducted to evaluate the relative efficacy (PASI 90 and PASI 100) and cost of biologic therapies approved or available in India for moderate-to-severe PsO. The analysis used cost per Number Needed to Treat (NNT), derived through a network meta-analysis (NMA), a validated statistical method that combines direct and indirect evidence to compare multiple drugs and generate comparative efficacy estimates.1
The biologics evaluated in the study included IL-17A inhibitors (ixekizumab and secukinumab), TNF inhibitors (adalimumab, etanercept and infliximab), and the anti-IL-12/23 (ustekinumab). The time points considered for the analysis were 12 weeks (induction therapy), 24, 52 (1 year), and 104 weeks (2 years) after therapy initiation. 1
The doses considered for each biologic at these time points were based on the approved dosing schedules. (Table 1)
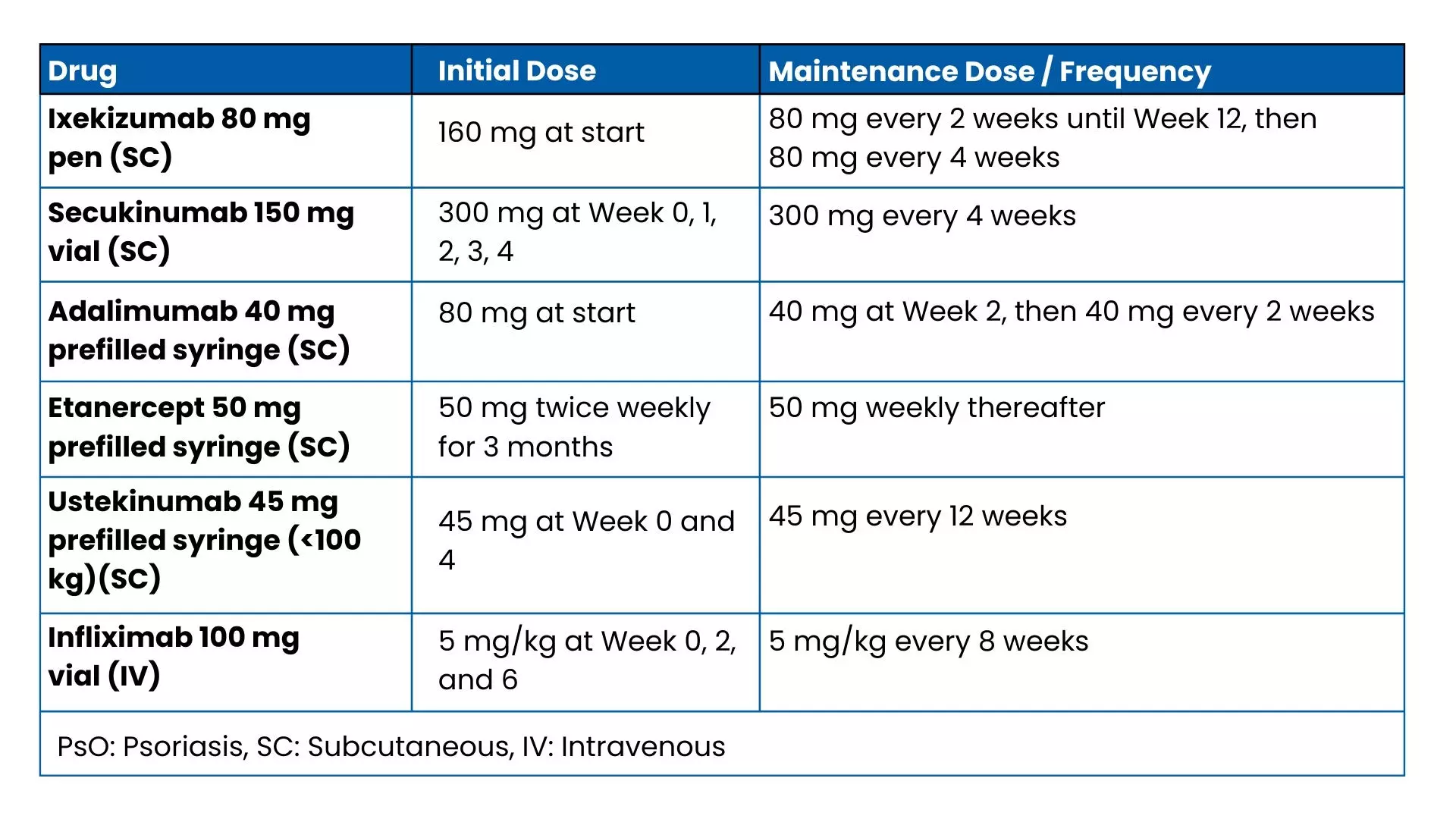
Table 1: Dosing schedule of the biologics used for treating PsO considered in the study
The Biologic cost was calculated as the number of doses required to be taken until the specified time points, multiplied by the maximum retail price (MRP) of the drug in India in September 2024. NNT indicates how many patients must receive a biologic to achieve one additional PASI90 or PASI100 response compared with placebo. The NMA calculated the NNTs at the end of 12 weeks as:
| NNT = 1 / (probability of response with biologic − probability of response with placebo) |
The cost of biologic per NNT calculated by multiplying the NNT of a drug with the corresponding treatment cost, the MRP per NNT of each biologic was obtained.
| Cost per NNT of a drug at a given time point = NNT of the drug at that time point x no. of doses required x cost per dose of the drug |
The key findings from the study are:
- Cost of biologics per NNT for PASI100 response at MRP:
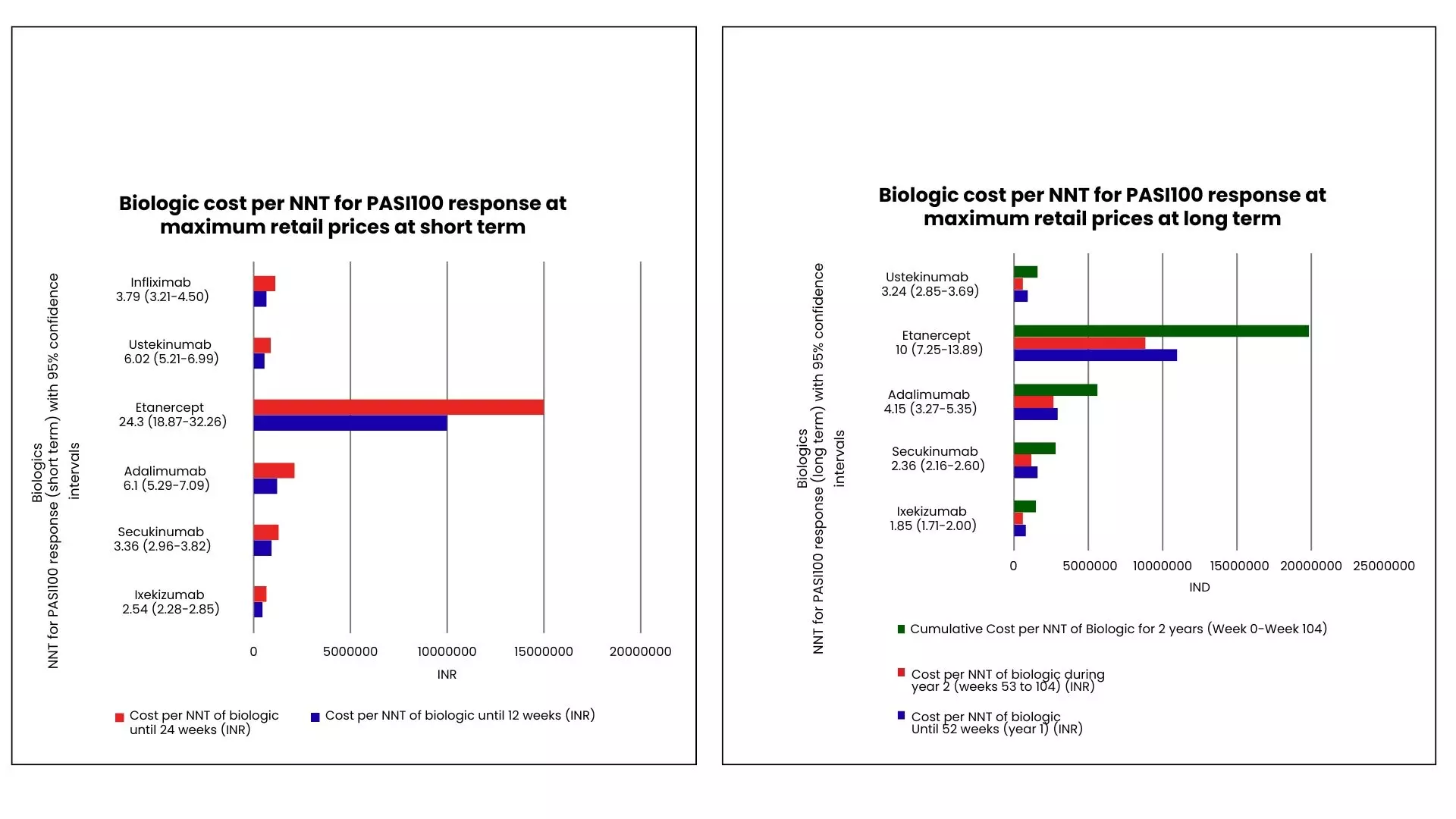
Ixekizumab demonstrated the lowest NNT based on MRP for achieving PASI100 in both short- and long-term assessments, resulting in the lowest cost per NNT at all time points, followed by ustekinumab. The cost advantage of ixekizumab over ustekinumab was 14% at 12 weeks, 24.4% at 24 weeks, 12% at 1 year, and 3.7% at 2 years. Over the full 2-year period, the cumulative cost per NNT for PASI100 remained lowest for ixekizumab, with a 9% advantage over ustekinumab. Etanercept showed the highest cost per NNT at every time point. (Figure 1)
- Cost of biologic per NNT for PASI90 response at MRP:
Ustekinumab had the lowest cost per NNT for PASI90 across all time points, followed by ixekizumab. Etanercept consistently showed the highest cost per NNT at all time points.*
*Adapted from <Sardana K et. al. A comparison of anti-IL-17A, anti TNF, and anti-IL-12/23 biologics for moderate-to-severe plaque psoriasis in India, based on cost per NNT and its effect on the therapeutic landscape and outcome. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_311_20251
Figure 1: Biologic cost per NNT for PASI100 response at MRPs at short term and long term
Among approved biologics based on MRP for moderate-to-severe PsO in India, ixekizumab shows the lowest cost per NNT for achieving PASI100 during induction and up to 2 years of maintenance. Analysis at the prices considered in the study indicates that ixekizumab offers the highest relative efficacy based on cost per NNT, although future cost changes and biosimilars may influence this. These insights may help public health agencies and dermatologists estimate short- and long-term treatment costs when selecting advanced therapies.
The study has a few limitations. NNT values were sourced from a previously published NMA, and cost-per-NNT analyses, being based on RCT data, may not fully reflect real-world influences such as adherence. Laboratory and clinic visit costs for adverse-event monitoring were not included. NNT values were extrapolated from 12–16 weeks to 24 weeks and from 44–52 weeks to 104 weeks due to the absence of published data at these time points. Cost per NNT for ustekinumab in adults weighing > 100 kg was not evaluated. Administration costs were also excluded, which is particularly relevant for infliximab due to its intravenous route. Additionally, biologic prices fluctuate with market dynamics, brand pricing, and biosimilar availability, which may influence overall cost estimates.
Reference: Sardana K, Neema S, Sapra D, Jagadeesan S, Muddebihal A, KB Rakesh, et al. A comparison of anti-IL-17A, anti TNF, and anti-IL-12/23 biologics for moderate-to-severe plaque psoriasis in India, based on cost per NNT and its effect on the therapeutic landscape and outcome. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_311_2025
PP-IX-IN-0989 | 05/01/2026
Eli Lilly and Company (India) Private Limited
For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001, India Ph.: +91-124-4753000/01 | www.lilly.com/in
For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com | For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com.
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. The information provided in this section is intended solely for the use of registered medical practitioner. This material is being provided to healthcare professionals only for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.

© 2026 Eli Lilly and Company


