- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Extra-Strength Jeuveau achieves positive outcomes for treatment of glabellar lines in adults for up to 6 months
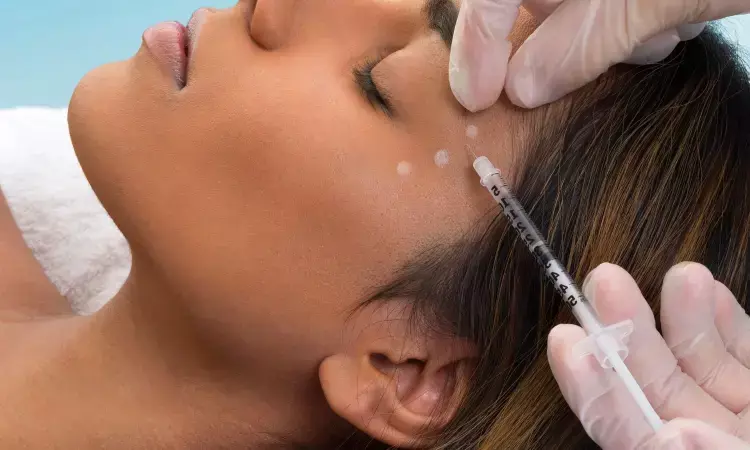
Evolus, Inc. recently revealed results from a Phase 2 clinical study showcasing the efficacy of the "extra-strength" 40U dose of Jeuveau in extending the duration of its effects. The study, presented at the 2023 American Society for Dermatologic Surgery (ASDS) Annual Meeting, demonstrated a remarkable 26 weeks, or 6 months, of duration with the higher dosage, reinforcing the potential for longer-lasting aesthetic outcomes.
The Phase 2 study compared the "extra-strength" formulation of 40U Jeuveau® to active controls, including the approved 20U of Jeuveau® and 20U of Botox®. Evaluation criteria included glabellar lines at maximum frown using the validated 4-point Glabellar Line Scale (GLS). The study examined various metrics, such as the time for patients to return to their baseline GLS score after treatment, the duration of effect for patients with at least a one-point GLS improvement, and the time for a patient to return to their baseline using the Global Aesthetic Improvement Scale.
The study reported a favorable and comparable safety profile for both arms of Jeuveau®. Approximately 88.9% of adverse events were rated as mild, with no serious adverse events observed across all three arms.
Investigator John Joseph, M.D., Facial Plastic Surgeon, expressed optimism about the findings, highlighting that the "extra-strength" formulation exhibited consistent longer-lasting effects of up to 26 weeks. Rui Avelar, M.D., Chief Medical Officer and Head of Research and Development at Evolus, emphasized the importance of the study's contribution to understanding the impact of increasing dose on extended duration.
Jeuveau® is the only neurotoxin exclusively dedicated to aesthetics, approved for the temporary improvement of moderate to severe vertical lines between the eyebrows (glabellar lines) in adults below 65 years of age. The "Extra-Strength" Glabellar Line Study, part of the TRANSPARENCY Clinical Program, has added valuable insights into achieving longer-lasting results with Jeuveau®.
The positive results from the Phase 2 trial pave the way for further exploration and potential regulatory approvals for the "extra-strength" formulation of Jeuveau®. Health care providers and patients alike may find this data valuable in decision-making regarding aesthetic treatments.
Reference:
Avelar, R. (n.d.). Jeuveau extra-strength longer duration phase II study first interim analysis. Q4cdn.com. Retrieved November 9, 2023, from https://s29.q4cdn.com/603291515/files/doc_events/2023/Jan/30/updated/2023.01.30-analyst-call-extra-strength-phase-ii-study-interim-results-website.pdf
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


