- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA approves dupilumab as first and only treatment Indicated for Prurigo Nodularis
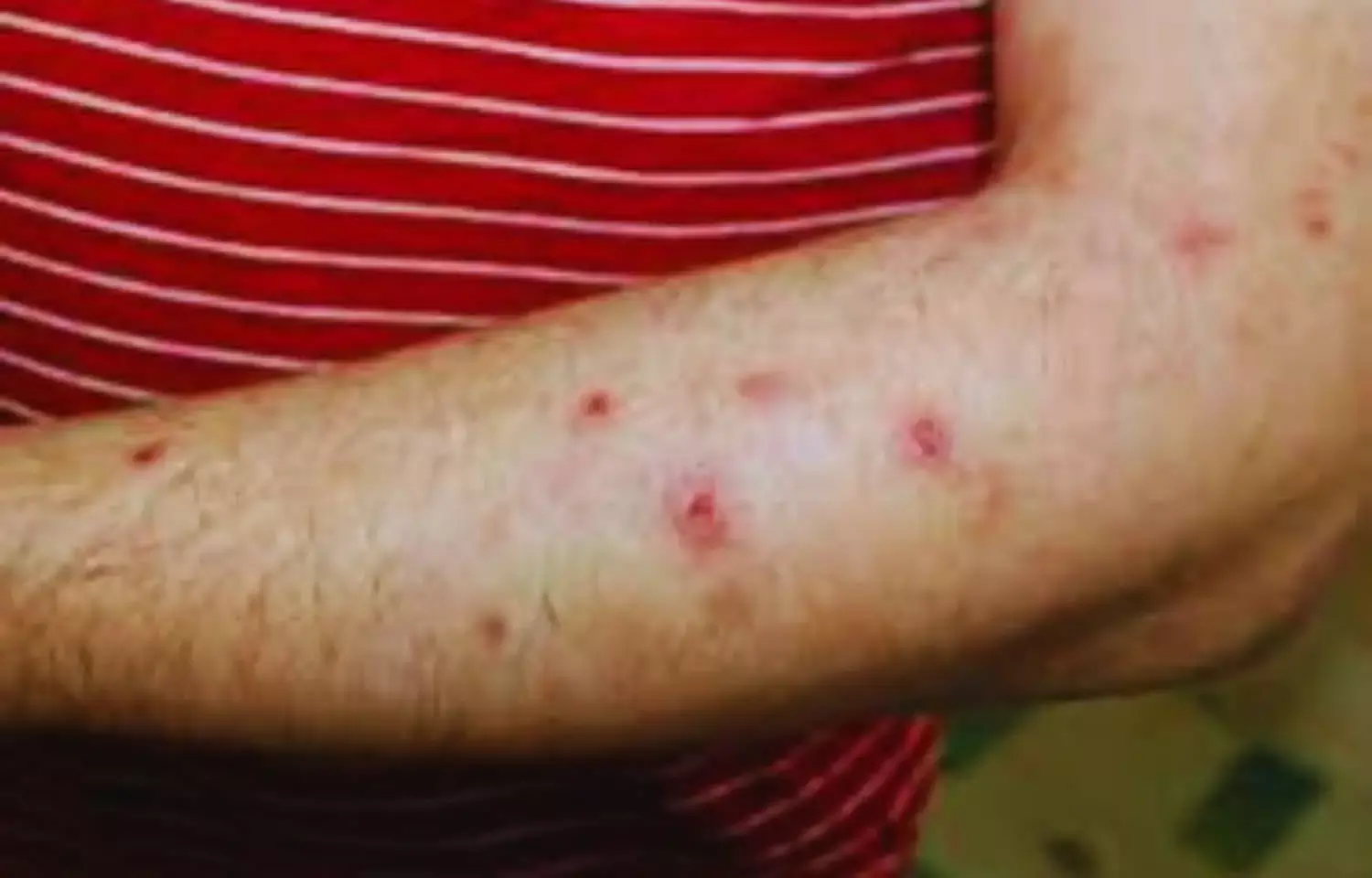
Prurigo nodularis is a chronic, debilitating skin disease with underlying type 2 inflammation and its impact on quality of life is one of the highest among inflammatory skin diseases.
The Food and Drug Administration (FDA) has approved Dupixent (dupilumab) as first and only treatment Indicated for Prurigo Nodularis for adult patients.
The FDA evaluated the Dupixent application for prurigo nodularis under Priority Review, which is granted to therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions.
"Patients living with prurigo nodularis must often contend with dozens, if not hundreds, of itchy and painful nodules covering their body and have not had an approved treatment option for their disease," said George D. Yancopoulos, M.D., Ph.D., President and Chief Scientific Officer at Regeneron, and a principal inventor of Dupixent.
"Dupixent has already transformed the treatment landscape of several diseases driven by type 2 inflammation-including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis-and been prescribed to more than half a million patients around the world for its approved indications. With this approval, those suffering with prurigo nodularis finally have a medicine to address the debilitating signs and symptoms of the disease."
"Until today, there were limited treatment options to manage the relentless itch and associated sensations of burning and stinging skin that can negatively impact the lives of patients struggling with prurigo nodularis," said Naimish Patel, M.D, Head of Global Development, Immunology and Inflammation at Sanofi.
"Dupixent has the potential to transform the standard-of-care for prurigo nodularis patients by alleviating the key hallmarks of the disease, such as reducing itch and achieving clearer skin. With Dupixent now approved in two diseases in dermatology where type 2 inflammation is a central driver, we look forward to further evaluating the potential of inhibiting IL-4 and IL-13 in other chronic skin diseases."
The FDA approval is based on data from two Phase 3 trials, PRIME and PRIME2, evaluating the efficacy and safety of Dupixent in adults with prurigo nodularis. Efficacy in these trials assessed the proportion of subjects with clinically meaningful reduction in itch, clearing of skin, or both:
- About three times as many Dupixent patients (60% and 58%) experienced a clinically meaningful reduction in itch from baseline at 24 weeks, compared to 18% and 20% for placebo, the primary endpoint in PRIME
- 44% and 37% of Dupixent patients experienced a clinically meaningful reduction in itch from baseline at 12 weeks, compared to 16% and 22% for placebo, the primary endpoint in PRIME2
- More than twice as many Dupixent patients (48% and 45%) achieved clear or almost clear skin at 24 weeks, compared to 18% and 16% for placebo
- More than three times as many Dupixent patients (39% and 32%) experienced both a clinically meaningful reduction in itch and clear or almost clear skin, compared to 9% and 9% of placebo patients at 24 weeks
The safety results of the trial were generally consistent with the known safety profile of Dupixent in its approved dermatology indication. The most common adverse events (≥2%) from pooled PRIME and PRIME2 data more frequently observed with Dupixent than placebo were nasopharyngitis (5% Dupixent, 2% placebo), conjunctivitis (4% Dupixent, 1% placebo), herpes infection (3% Dupixent, 0% placebo), dizziness (3% Dupixent, 1% placebo), muscle pain (3% Dupixent, 1% placebo), and diarrhea (3% Dupixent, 1% placebo).
A regulatory filing for prurigo nodularis is under review by the European Medicines Agency, and submissions to regulatory authorities in additional countries are also planned in 2022.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


