- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
IDP-126 effective triple-combination therapy for acne treatment in children and adolescents, study finds
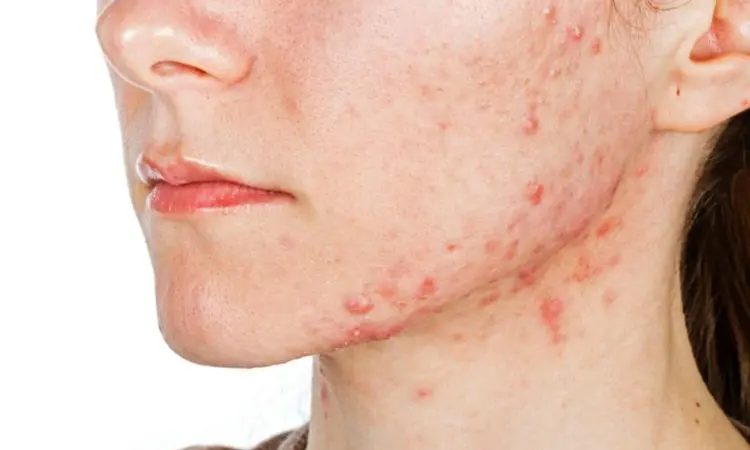
USA: The fixed-combination IDP-126 gel demonstrated promising results as the first triple-combination product for acne treatment in children and adolescents aged 9 to 17 years, a recent study published in Pediatric Dermatology has shown. IDP-126 achieved significantly greater success rates than dyad combinations and vehicle.
Topical clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel (IDP-126) showed superior efficacy to dyad component and vehicle in pediatric patients with moderate-to-severe acne, with over half of IDP-126-treated trial patients achieving treatment success. IDP-126 was well-tolerated and significantly improved participants' quality of life assessments.
"The safety and efficacy profile of IDP-126—the only fixed-combination acne medication in development containing three recommended treatments for once-daily use—shows its potential as an option for effective acne treatment," the researchers wrote.
In the post hoc analysis of a phase 2 trial, Lawrence F. Eichenfield, San Diego School of Medicine, La Jolla, California, USA, and colleagues investigated the safety and efficacy of IDP-126 in adolescents and children with moderate-to-severe acne.
Participants ≥9 years of age with moderate-to-severe acne were eligible in a randomized, double-blind phase 2 study. They were randomized in the ratio of 1:1:1:1:1 for 12 weeks to once-daily IDP-126, one of three dyad combination gels, or vehicle gel.
The post hoc analysis of 394 pediatric participants included children and adolescents up to 17. Assessments included noninflammatory/inflammatory lesion counts, treatment success, Acne-QoL (Acne-Specific Quality of Life) questionnaire, cutaneous safety/tolerability, and treatment-emergent adverse events (TEAEs).
The study revealed the following findings:
- At Week 12, treatment success rates were significantly greater with IDP-126 (55.8%) than with vehicle (5.7%;) or any of the dyad combinations (range: 30.8%–33.9%).
- Lesion reductions with IDP-126 were also significantly greater than with vehicle (inflammatory: 78.3% versus 45.1%; noninflammatory: 70.0% versus 37.6%) and 9.2%–16.6% greater than with any of the dyad combinations.
- Increases (improvements) from baseline in Acne-QoL domain scores were generally more significant with IDP-126 than in any other treatment group.
- The most common treatment-related TEAEs across treatment groups were application site pain and dryness. Most treatment-related TEAEs were of mild-to-moderate severity.
Results from the post hoc analysis showed that pediatric participants treated with IDP-126, a novel fixed-dose triple-combination clindamycin phosphate 1.2%/BPO 3.1%/adapalene 0.15% polymeric gel, experienced rapid reductions in noninflammatory and inflammatory lesion counts and treatment success rates of over 50%, higher than for those treated with vehicle or any of three dyad combination gels.
Reference:
Eichenfield LF, Stein Gold L, Kircik LH, Werschler WP, Beer K, Draelos ZD, Tanghetti EA, Papp KA, Baldwin H, Lain E, Sadick N, Gooderham MJ, Konda A. Triple-combination clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel for moderate-to-severe acne in children and adolescents: Randomized phase 2 study. Pediatr Dermatol. 2023 Mar 22. doi: 10.1111/pde.15283. Epub ahead of print. PMID: 36949579.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


