- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
IV fosphenytoin appears to be a promising alternative treatment for acute zoster-associated pain: Study
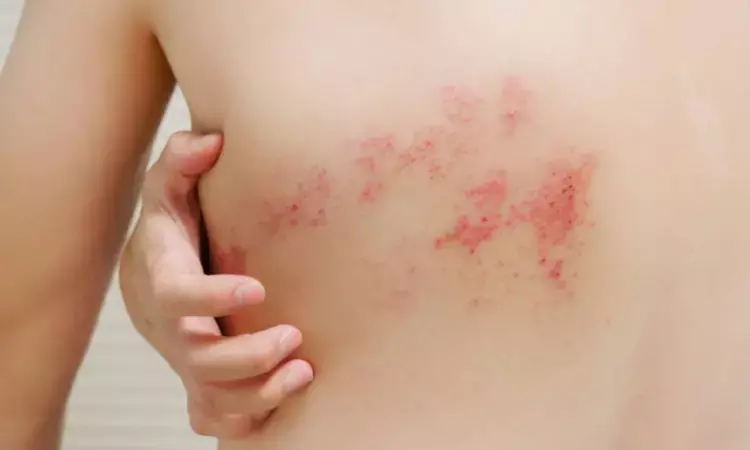
Japan: A placebo-controlled randomized trial published in The Journal of Dermatology has shed light on the safety and efficacy of intravenous (IV) fosphenytoin for patients with acute herpes zoster-associated pain (ZAP).
The researchers revealed a rapid analgesic effect of a single dose of IV fosphenytoin for acute ZAP in patients with HZ for whom nonopioid drugs had shown an insufficient analgesic effect. "Therefore, IV fosphenytoin is promising as a potential alternative treatment for acute ZAP," they wrote.
Herpes zoster (HZ) is a common viral disease caused by reactivation of the varicella-zoster virus, which lies latent in the dorsal root ganglia of the cranial and spinal nerves after primary varicella-zoster virus infection. Most patients with herpes zoster develop acute zoster-associated pain. Nonopioid analgesics are usually used for treating acute ZAP but are frequently ineffective.
Masako Iseki, Department of Anesthesiology and Pain Medicine, Juntendo University Faculty of Medicine, Bunkyo-ku, Tokyo, Japan, and colleagues administered IV fosphenytoin, the prodrug of phenytoin, to patients with acute ZAP to investigate its analgesic safety and efficacy.
They conducted a phase II, double-blind, placebo-controlled, randomized trial at 13 medical institutions in Japan of IV fosphenytoin in Japanese inpatients with acute zoster-associated pain for whom nonopioid analgesics had shown an insufficient analgesic effect.
The patients were randomly assigned in a 1:1:1 ratio to receive a single IV dose of fosphenytoin at 18 mg/kg (high dose), a single intravenous dose of fosphenytoin at 12 mg/kg (low dose), or a placebo.
The study's primary endpoint was the mean change per hour (slope) in the numerical rating scale score from the baseline score until 120 min following the dosing.
The study led to the following findings:
- Seventeen patients were assigned randomly to the low-dose fosphenytoin group (n = 6, median age 62.5 years), high-dose fosphenytoin group (n = 5, median age 69.0 years), and placebo group (n = 5, median age 52.0 years). One patient was excluded because of investigational drug dilution failure.
- This study was discontinued due to the influences of coronavirus disease 2019.
- The slope was significantly lower in the high- and low-dose fosphenytoin groups than in the placebo group.
- Responsiveness to intravenous fosphenytoin (≥2-point reduction in the numerical rating scale score from baseline to 120 min after dosing) was inferred at plasma total phenytoin concentrations of 10–15 μg/mL.
- Treatment-emergent adverse events caused no safety concerns in the clinical setting, and intravenous fosphenytoin was well tolerated.
"Intravenous fosphenytoin appears to be a promising and effective alternative treatment for acute zoster-associated pain," the researchers wrote. "A further confirmatory clinical study is required."
Reference:
Iseki, M., Yamamoto, T., Ogawa, Y., Majima, Y., Abe, Y., Watanabe, D., Amaya, F., Hasegawa, T., Inafuku, K., Kosugi, T., Nomura, Y., Deguchi, T., Hamada, T., Shimizu, K., Arai, S., Takahashi, M., Hamada, I., Ishikawa, Y., & Kawashima, M. (2024). Efficacy and safety of intravenous fosphenytoin for patients with acute herpes zoster-associated pain: A placebo-controlled randomized trial. The Journal of Dermatology, 51(2), 234-242. https://doi.org/10.1111/1346-8138.17054
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


