- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Low-dose minocycline hydrochloride effective in the treatment of papulopustular rosacea: JAMA
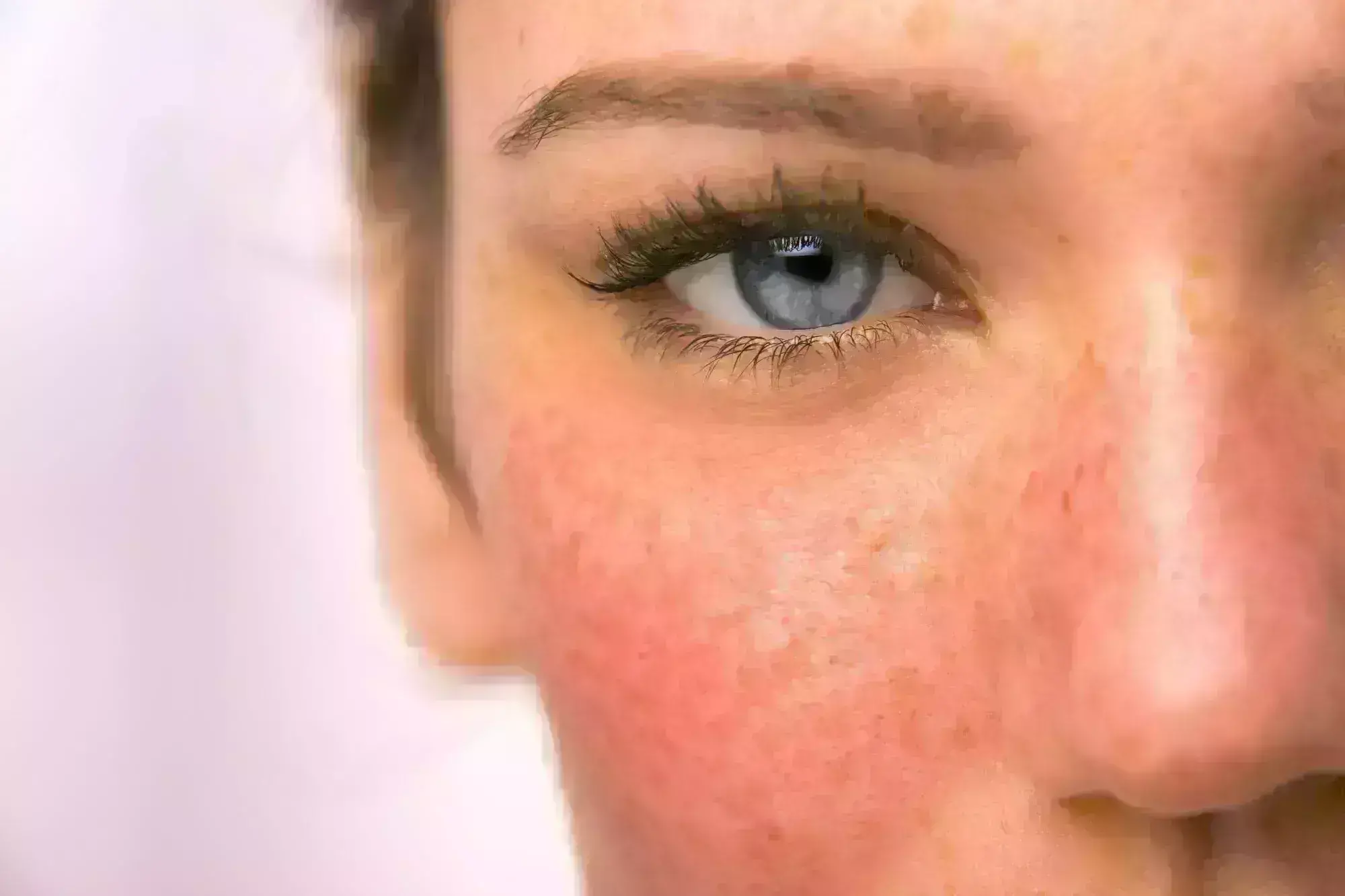
A new study published in the Journal of American Medical Association showed that patients with moderate to severe papulopustular rosacea responded better to a low-dose (40 mg) modified formulation of minocycline hydrochloride (DFD-29) than doxycycline and placebo.
Papulopustular rosacea (PPR) is being treated with DFD-29, a low-dose modified formulation of minocycline hydrochloride. When treating papulopustular rosacea, minocycline hydrochloride modified release capsules, 40 mg (DFD-29), have demonstrated a notable therapeutic advantage over doxycycline and a placebo. Yet, the effectiveness and impact are not fully documented. Thus, to ascertain the safety and effectiveness of DFD-29, 40 mg, in treating PPR, Neal Bhatia and colleagues carried out this investigation in comparison to doxycycline, 40 mg, and a placebo.
Data from 2 phase 3 randomized clinical studies (MVOR-1 and MVOR-2) from 61 US and German locations between March 2022 and May 2023 were included in this analysis. Included were healthy persons with mild to severe PPR who were at least 18 years old. For 16 weeks, the participants were randomized 3:3:2 to receive 40 mg of oral DFD-29 (minocycline hydrochloride capsules), 40 mg of doxycycline, or a placebo once daily.
The proportion of individuals who had treatment success on the Investigator's Global Assessment (IGA) with DFD-29 compared to placebo and the reduction in the total number of inflammatory lesions with DFD-29 compared to placebo were the coprimary efficacy outcomes. Comparisons between DFD-29 and doxycycline in coprimary outcomes and between DFD-29 and a placebo in erythema reduction were examples of secondary outcomes.
MVOR-1 had 323 randomized individuals, whereas MVOR-2 involved 330 randomized participants. IGA success rates showed that DFD-29 was more effective than both doxycycline and a placebo. Also, DFD-29 outperformed doxycycline and placebo in terms of least-squares mean decreases in total inflammatory lesions.
MVOR-1 reported 32 of 121 (26.4%), 25 of 116 (21.6%), and 27 of 76 (35.5%) adverse events with DFD-29, doxycycline, and placebo, whereas MVOR-2 reported 40 of 121 (33.1%), 51 of 122 (41.8%), and 30 of 82 (36.6%) adverse events.
The most frequent side effects with DFD-29, doxycycline, and placebo were COVID-19, which was reported in 4 of 121 (3.3%), 3 of 116 (2.6%), and 4 of 76 (5.3%) in MVOR-1 and 7 of 122 (5.7%), 8 of 121 (6.6%), and 5 of 82 (6.1%) in MVOR-2. Also, nasopharyngitis was reported in 4 of 121 (3.3%), 2 of 116 (1.7%), and 3 of 76 (3.9%) in MVOR-1 and 13 of 122 (10.7%), in MVOR-2, respectively. Overall, in treating PPR, DFD-29 showed a good risk-benefit balance and was more effective than both doxycycline and a placebo.
Source:
Bhatia, N., Del Rosso, J., Stein Gold, L., Lain, E., Draelos, Z. D., Sidgiddi, S., & MVOR-1 and MVOR-2 Study Investigators. (2025). Efficacy, safety, and tolerability of oral DFD-29, a low-dose formulation of minocycline, in Rosacea: Two phase 3 randomized clinical trials. JAMA Dermatology (Chicago, Ill.). https://doi.org/10.1001/jamadermatol.2024.6542
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


