- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Oral IL-23 inhibitor promising for treatment of moderate-to-severe plaque psoriasis: NEJM
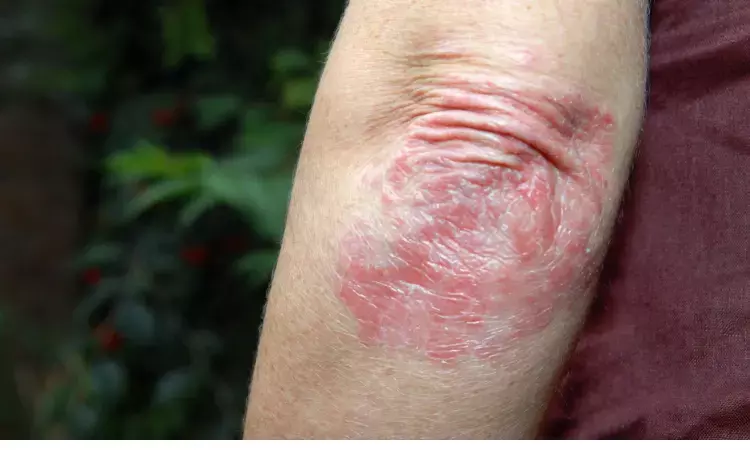
Canada: A recent study showed greater treatment efficacy with the interleukin-23-receptor antagonist peptide JNJ-77242113 after 16 weeks of once- or twice-daily oral administration than placebo in patients with moderate-to-severe plaque psoriasis. The findings from the FRONTIER 1, a phase 2 dose-finding trial, were published online in The New England Journal of Medicine.
"The level of reduction of psoriasis that was observed with higher doses of JNJ-77242113 at week 16 was similar in magnitude to the responses seen with several of the injectable biologics that are currently approved for psoriasis," investigators in the FRONTIER 1 trial wrote.
Psoriasis is an immune-mediated, multisystemic, inflammatory disease that predominantly involves the skin and joints. The disease is also a risk factor for atherosclerotic cardiovascular disease. Interleukin-23 plays an important role in pathogenic T-cell activation in psoriasis.
Using monoclonal antibodies has changed the treatment of various immune-mediated inflammatory diseases, including psoriasis. However, these large proteins must be administered via injection. Many patients prefer oral treatments over injections, and injections are especially problematic among children and among patients with a fear of needles. Thus, there is a need for efficacious targeted therapies that can be administered orally.
JNJ-77242113 is a novel, orally administered interleukin-23–receptor antagonist peptide selectively blocks interleukin-23 signalling and downstream cytokine production. Robert Bissonnette, University of Toronto, Toronto, Canada, and colleagues report the results of FRONTIER 1, a phase 2 trial of JNJ-77242113 in patients with moderate-to-severe plaque psoriasis.
In the trial, 255 patients with moderate-to-severe plaque psoriasis were randomly assigned to receive JNJ-77242113 at a dose of 25 mg once daily, 25 mg twice daily, 50 mg once daily, 100 mg once daily, or 100 mg twice daily or placebo for 16 weeks. The study's primary endpoint was a reduction from a baseline of at least 75% in the Psoriasis Area and Severity Index (PASI) score (PASI 75 response). PASI scores range from 0 to 72, with higher scores implying greater extent or severity of psoriasis) at week 16.
The mean PASI score at baseline was 19.1. The mean psoriasis duration was 18.2 years, and 78% of the patients across all the trial groups had previously received systemic treatments.
The researchers reported the following findings:
- At week 16, the percentages of patients with a PASI 75 response were higher among those in the JNJ-77242113 groups (37%, 51%, 58%, 65%, and 79% in the 25-mg once-daily, 25-mg twice-daily, 50-mg once-daily, 100-mg once-daily, and 100-mg twice-daily groups, respectively) than among those in the placebo group (9%), a finding that showed a significant dose–response relationship.
- The most common adverse events included coronavirus disease 2019 (in 12% of the patients in the placebo group and 11% of those across the JNJ-77242113 dose groups) and nasopharyngitis (in 5% and 7%, respectively).
- The percentages of patients who had at least one adverse event were similar in the combined JNJ-77242113 dose group (52%) and the placebo group (51%).
- There was no evidence of a dose-related increase in adverse events across the JNJ-77242113 dose groups.
"In patients with moderate-to-severe plaque psoriasis, JNJ-77242113 showed a dose-response relationship and greater efficacy than placebo, as measured by the PASI 75 response at week 16," the researchers wrote. "Overall, we found no evidence of a relationship between the JNJ-77242113 dose and the incidence of adverse events."
Limitations include a small number of patients in each trial group and the short duration of treatment. In addition, no corrections were made for multiplicity, so the researchers could not infer definitive effects of JNJ-77242113 for particular dose groups or secondary endpoints.
Reference:
Bissonnette R, Pinter A, Ferris LK, Gerdes S, Rich P, Vender R, Miller M, Shen YK, Kannan A, Li S, DeKlotz C, Papp K. An Oral Interleukin-23-Receptor Antagonist Peptide for Plaque Psoriasis. N Engl J Med. 2024 Feb 8;390(6):510-521. doi: 10.1056/NEJMoa2308713. PMID: 38324484.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


