- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Piclidenoson efficacious and safe in the treatment of patients with plaque psoriasis: Study
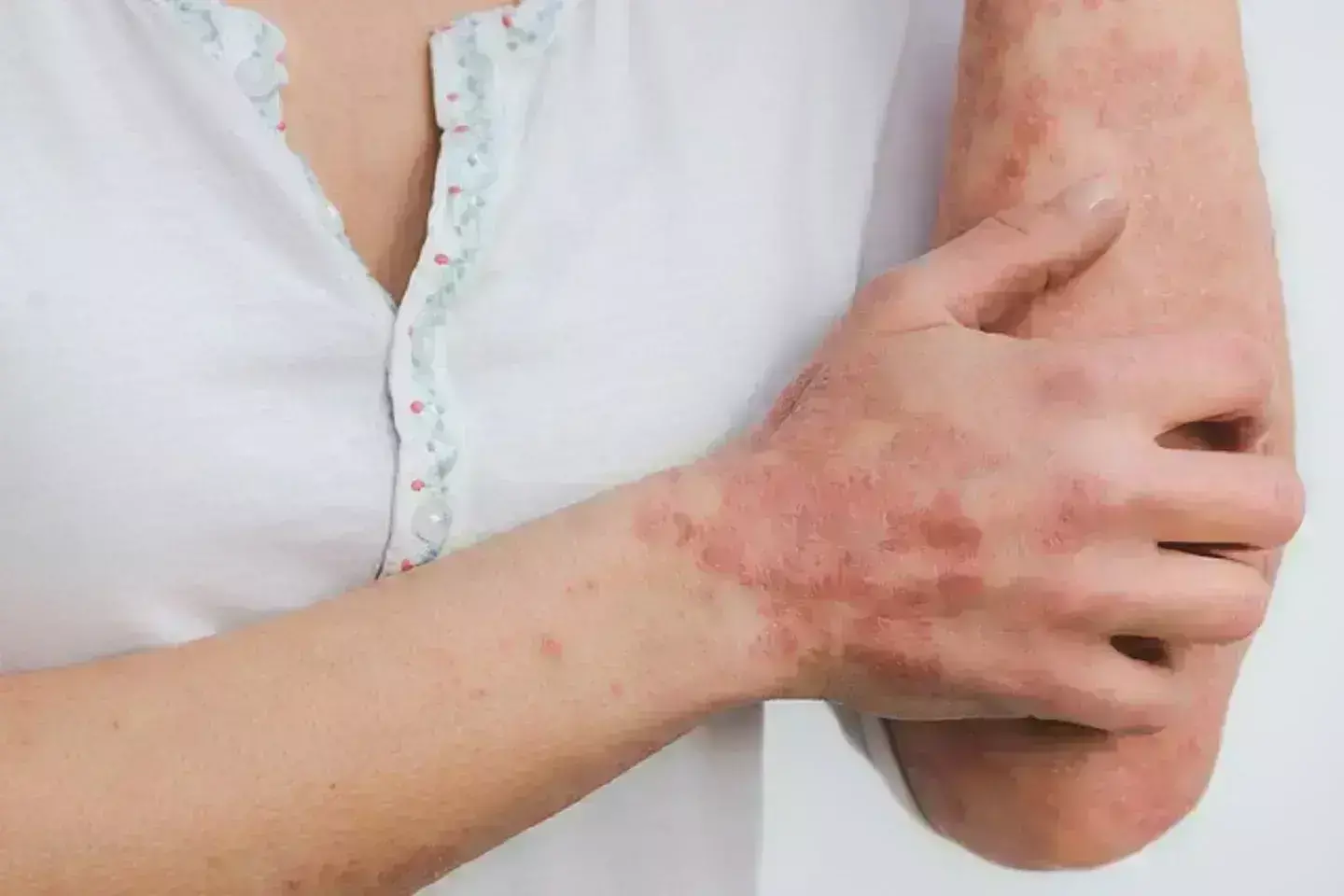
Piclidenoson is efficacious and safe in the treatment of patients with plaque psoriasis suggests a study published in the Journal of the European Academy of Dermatology and Venereology.
A3 adenosine receptor (A3AR) is overexpressed in the skin and peripheral blood mononuclear cells of psoriasis patients. We investigated the efficacy/safety of piclidenoson (CF101), an orally bioavailable A3AR agonist that inhibits IL-17 and IL-23 production in keratinocytes, in moderate-to-severe plaque psoriasis. The randomized, placebo- and active-controlled, double-blind phase 3 COMFORT-1 trial randomized patients (3:3:3:2) to piclidenoson 2 mg BID, piclidenoson 3 mg BID, apremilast 30 mg BID or placebo. At Week 16, patients in the placebo arm were re-randomized (1:1:1) to piclidenoson 2 mg BID, piclidenoson 3 mg BID or apremilast 30 mg BID. The primary endpoint was the proportion of patients achieving ≥75% improvement in Psoriasis Area and Severity Index (PASI) from baseline (PASI-75) at Week 16 versus placebo.
Results: A total of 529 patients were randomized and received ≥1 dose of study medication (safety population). The efficacy analysis population for the primary endpoint included 426 patients (piclidenoson 2 mg BID, 127; piclidenoson 3 mg BID, 103; apremilast, 118; placebo, 78). Piclidenoson at 2 and 3 mg BID exhibited similar efficacy. The primary endpoint was met with the 3 mg BID dose: PASI 75 rate of 9.7% versus 2.6% for piclidenoson versus placebo, p = 0.037. The PASI responses with piclidenoson continued to increase throughout the study period in a linear manner. At week 32, analysis in the per-protocol population showed that a greater proportion of patients in the piclidenoson 3 mg BID arm (51/88, 58.0%) achieved improvement from baseline in Psoriasis Disability Index (PDI) compared to apremilast (59/108, 55.1%), and the test for noninferiority trended towards significance (p = 0.072). The safety/tolerability profile of piclidenoson was excellent and superior to apremilast. Piclidenoson demonstrated efficacy responses that increased over time alongside a favourable safety profile.
Reference:
Papp KA, Beyska-Rizova S, Gantcheva ML, Slavcheva Simeonova E, Brezoev P, Celic M, et al. Efficacy and safety of piclidenoson in plaque psoriasis: Results from a randomized phase 3 clinical trial (COMFORT-1). J Eur Acad Dermatol Venereol. 2024; 38: 1112–1120. https://doi.org/10.1111/jdv.19811
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


