- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Pimecrolimus use in Asian infants with atopic dermatitis not associated cancer risk
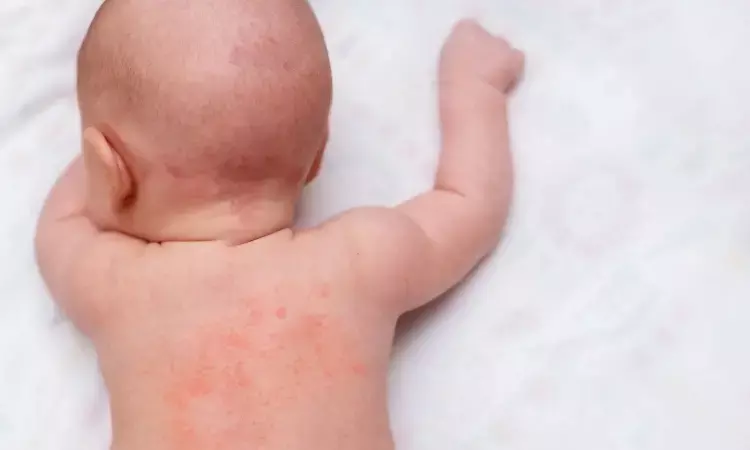
Taiwan: Based on the available evidence, an expert panel has recommended pimecrolimus (PIM) in infants with atopic dermatitis (AD) aged three months to 2 years in the Asian population in a recent study published in Dermatology and Therapy. They suggest that labelling restrictions of pimecrolimus in this infant population are no longer justified.
Existing meta-analyses and post-marketing surveys did not reveal an increased cancer risk with long-term pimecrolimus use, and hence is a safe and effective treatment for the long-term management of atopic dermatitis in infants.
"Atopic dermatitis treatment, based on the clinical evidence, should be initiated as early as infancy," the Asian Expert Panel led by Chia-Yu Chu from the National Taiwan University Hospital and National Taiwan University College of Medicine in Taipei, Taiwan, wrote. "We recommend that regulatory authorities in Asian countries remove the current boxed warnings, as this will ensure that AD patients have access to effective medications with comprehensively established safety profiles." The current boxed warning is due to the lack of long-term safety data and the potential risk of malignancies development.
Atopic dermatitis is a common chronic, multisystem inflammatory skin disease in pediatric patients. In the Asia–Pacific region, there has been an increase in the prevalence of AD. Studies have revealed that epigenetic, genetic, cultural and environmental factors may contribute to differences in the prevalence and clinical manifestation of AD between races. Early AD treatment is necessary to prevent the condition from leading to comorbidities such as allergic rhinitis and asthma.
Topical corticosteroids (TCS) are used as first-line therapy for AD treatment, but long-term use poses a risk to a patient's health. Pimecrolimus (1%) is a topical calcineurin inhibitor indicated for treating mild to moderate atopic dermatitis. Compared to TCS, Pimecrolimus has no apparent increase in adverse events and causes less burning sensation than tacrolimus.
Several clinical trials have established pimecrolimus's safety and efficacy, yet in many Asian countries, pimecrolimus use in infants is still restricted owing to safety concerns.
The panel noted that early treatment of AD is essential to prevent worsening, decrease the significant burden of AD on the entire society and family and prevent the development of atopic comorbidities.
PIM use has advantages, including a mean EASI reduction, reduced flares risk, early treatment success, and long-term disease control. Post-marketing surveys revealed no increased cancer risks linked to TCI or TCS exposure. Pimecrolimus has already received approval from many Asian countries for treating atopic dermatitis in children.
To conclude, the expert panel recommends pimecrolimus in the Asia population in infants ages three months to 2 years.
Reference:
Chu, CY., Yao, TC., Shih, IH. et al. Pimecrolimus for the Treatment of Atopic Dermatitis in Infants: An Asian Perspective. Dermatol Ther (Heidelb) (2023). https://doi.org/10.1007/s13555-022-00886-9
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


