- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Rilzabrutinib could be effective treatment option in individuals with moderate to severe chronic spontaneous urticaria: JAMA
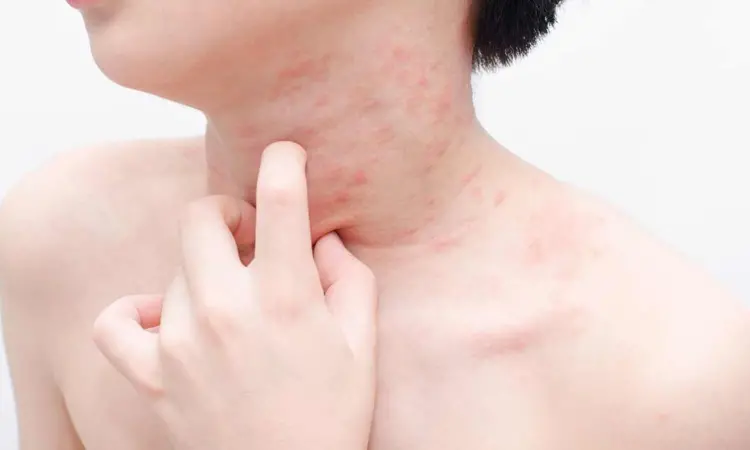
A new study published in the Journal of American Medical Association showed that Rilzabrutinib decreased itching and hives while preserving a positive risk-benefit profile, indicating that it might be a useful therapy for individuals with moderate to severe chronic spontaneous urticaria (CSU) that is resistant to antihistamines.
The primary cause of chronic spontaneous urticaria, a skin condition, is the activation of cutaneous mast cells via a variety of pathways. B cells and mast cells contain the protein bruton tyrosine kinase (BTK), which is essential for several immune-mediated disease processes. In order to ascertain the effectiveness and risk profile of rilzabrutinib, an oral, covalent, reversible, next-generation BTK inhibitor, in the treatment of patients with CSU, Ana Giménez-Arnau and colleagues carried out this investigation.
A 52-week phase 2 research, the Rilzabrutinib Efficacy and Safety in CSU (RILECSU) randomized clinical trial consisted of a 12-week dose-ranging, double-blind, placebo-controlled phase that was followed by a 40-week open-label extension. From November 24, 2021, until April 23, 2024, the trial was held in 12 countries. 51 centers across Asia, North America, Europe, and South America, recruited and randomly assigned individuals.
Adults with moderate to severe CSU who were not effectively managed with H1-antihistamine therapy, ranging in age from 18 to 80, were enrolled in the experiment. Patients were randomized 1:1:1:1 to 400 mg of rilzabrutinib, 400 mg once a day in the evening, 800 mg twice daily, 1200 mg three times daily, or a matched placebo. Change from baseline at week 12 in either UAS7 (for non-US reference nations) or ISS7 (for the US and US reference countries) was the main end objective.
A total of 160 responders who were either omalizumab-naive or omalizumab-incomplete were randomly assigned. Only the 143 individuals who had never used omalizumab were part of the primary analysis population. At week 12, ISS7 and UAS7 showed significant decreases with rilzabrutinib (1200 mg/d) compared to placebo from baseline. Improvements were also seen in the weekly Angioedema Activity Score (AAS7) and weekly Hives Severity Score (HSS7).
As early as week 1, ISS7, HSS7, UAS7, and AAS7 showed improvements. At week 12, CSU-related biomarkers, such as immunoglobulin (Ig)-G antithyroid peroxidase, soluble Mas-related G protein–coupled receptor X2, IgG anti-Fc-ε receptor 1, and interleukin-31, were lower than placebo. Rilzabrutinib had a good risk-benefit profile; headache, nausea, and diarrhea were more common side effects with rilzabrutinib than with a placebo.
Overall, the findings of this clinical study support that rilzabrutinib may be a useful BTKI with a good risk-benefit profile for treating H1-antihistamine-refractory patients with CSU.
Reference:
Giménez-Arnau, A., Ferrucci, S., Ben-Shoshan, M., Mikol, V., Lucats, L., Sun, I., Mannent, L., & Gereige, J. (2025). Rilzabrutinib in antihistamine-refractory chronic spontaneous urticaria: The RILECSU phase 2 randomized clinical trial. JAMA Dermatology (Chicago, Ill.). https://doi.org/10.1001/jamadermatol.2025.0733
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


