- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Rilzabrutinib effective and safe against chronic spontaneous urticaria, finds study
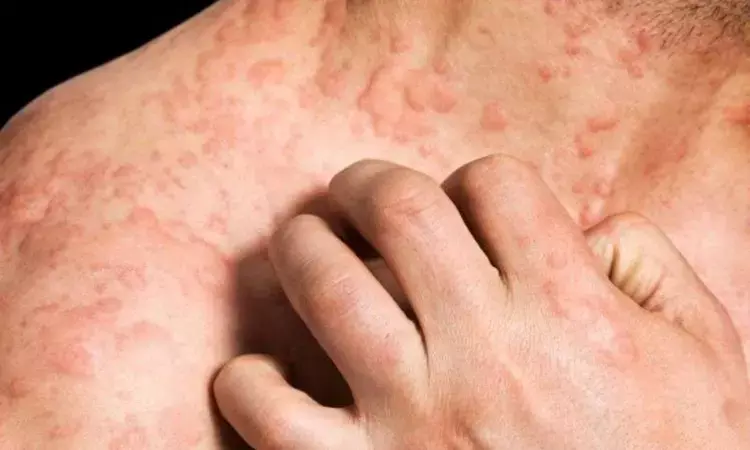
A recent study published in the Journal of Allergy and Clinical Immunology unveiled promising results from a Phase 2 study evaluating the efficacy and safety of rilzabrutinib (SAR444671) in addressing Chronic Spontaneous Urticaria (CSU). CSU is a distressing skin condition often resistant to conventional treatments, is characterized by recurring hives and intense itching, significantly impacting quality of life of the patients.
The RILECSU study encompassed a 52-week investigation involving adults with moderate-to-severe CSU that is inadequately managed by H1 antihistamine treatment alone. The study comprised a 12-week randomized, double-blind, placebo-controlled, dose-ranging phase that was succeeded by a 40-week open-label extension period.
The participants (N=160) were randomly assigned to receive rilzabrutinib at varying doses or a matching placebo. The individuals who were administered with rilzabrutinib at 400mg three times a day (TID) demonstrated significant improvements when compared to the placebo group. A marked reduction in weekly Itch Severity Score (ISS7) and Urticaria Activity Score (UAS7) was observed as early as Week 1, with sustained efficacy through Week 12.
Also, rilzabrutinib expressed a favorable safety profile, with adverse events that ranged from headache, nausea to diarrhea occurring at a higher frequency but remaining manageable. These findings highlight the potential of rilzabrutinib as a promising therapeutic option for the individuals with the debilitating effects of CSU.
This study emphasized the significance of rilzabrutinib's rapid onset and tolerability in addressing the unmet needs of CSU patients. The results pave the way for further exploration into the efficacy of rilzabrutinib across diverse patient populations and reinforce the importance of continued research in advancing treatment modalities for chronic skin diseases.
Source:
Maurer, M., Gimenez-Arnau, A., Ferrucci, S., Mikol, V., Sun, I., Mannent, L., & Gereige, J. (2024). Efficacy and Safety of Rilzabrutinib in Patients With Chronic Spontaneous Urticaria: 12-Week Results From the RILECSU Phase 2 Dose-Ranging Study. In Journal of Allergy and Clinical Immunology (Vol. 153, Issue 2, p. AB373). Elsevier BV. https://doi.org/10.1016/j.jaci.2023.11.893
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


