- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Ruxolitinib shows promise in managing atopic dermatitis: Study
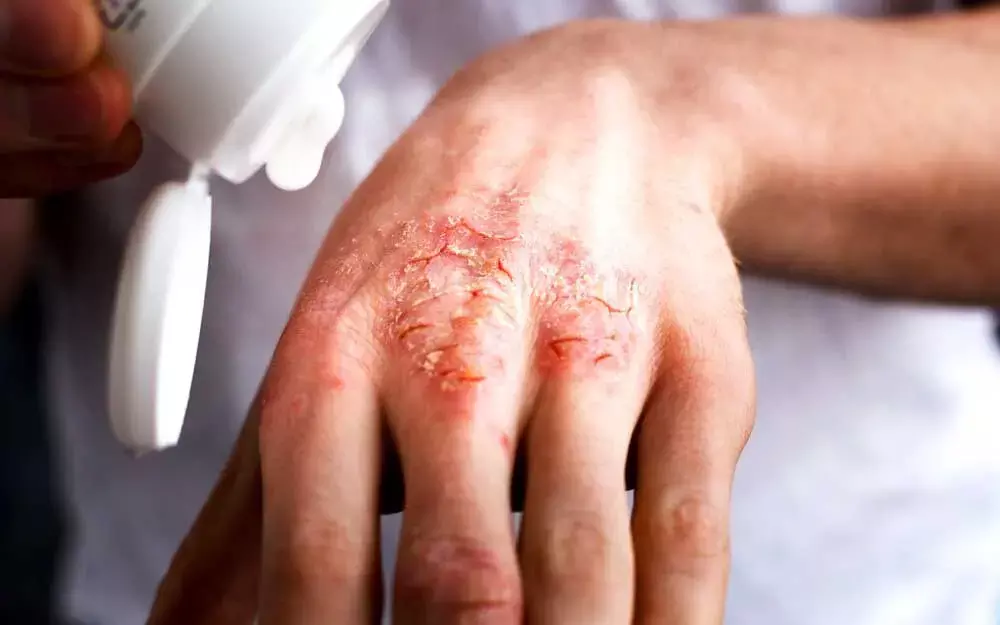
In a recent development, researchers have highlighted that Ruxolitinib (RUX) cream has shown anti-inflammatory and prompt antipruritic effects with superior efficacy versus vehicle and was well tolerated, in a phase 2 study in adults with atopic dermatitis (AD).Findings have been put forth in Journal of the American Academy of Dermatology.
Atopic dermatitis (AD) is a common, chronic, and relapsing inflammatory skin disease in which itch has a substantial and negative impact on the quality of life (QoL) of both patients and their caregivers. QoL burden in AD is similar to that of other chronic diseases, such as psoriasis and asthma, even in patients with mild to moderate disease.
Currently, the management of AD includes topical treatments such as emollients, corticosteroids, calcineurin inhibitors, and a recently approved phosphodiesterase 4 inhibitor (crisaborole) that are designed to restore skin barrier function and/or suppress inflammation.However, current topical therapies may be limited by local tolerability issues, restrictions for use on sensitive skin areas, and/or insufficient efficacy.
Ruxolitinib (RUX), a potent, selective inhibitor of JAK1 and JAK2, has a low molecular weight and is amenable to formulation in water-containing vehicles.it is well documented that Janus kinases (JAKs) mediate type 2 and other cytokine signaling involved in the pathogenesis of AD.In addition, JAK inhibition also occurs directly on sensory neurons and may also improve skin barrier function. Thus, JAK inhibitors are uniquely suited for the treatment of AD through their beneficial effect on inflammation and restoration of the epithelial barrier
So,researchers aimed to evaluate 8-week efficacy and safety in two phase 3 studies of RUX cream in patients with AD.For the study design, TRuE-AD1 (NCT03745638) and TRuE-AD2 (NCT03745651) enrolled patients aged ≥12 years with AD for ≥2 years, an IGA score of 2/3, and 3% to 20% affected body surface area. Patients were randomized 2:2:1 to twice-daily 0.75% RUX cream, 1.5% RUX cream, or vehicle cream for 8 continuous weeks. The primary endpoint was IGA treatment success (IGA-TS) at Week 8 (IGA score of 0/1 and ≥2-grade improvement from baseline).
Data analysis put forth some interesting facts.
- In TRuE-AD1/TRuE-AD2, 631/618 patients were randomized (631/577 analyzed for efficacy). Significantly more patients achieved IGA-TS with 0.75% RUX cream (50.0%/39.0%) and 1.5% RUX cream (53.8%/51.3%) versus vehicle (15.1%/7.6%; P<0.0001) at Week 8.
- Significant itch reductions versus vehicle were reported within 12 hours of first application of 1.5% RUX (P<0.05).
- Application site reactions were infrequent (<1%) and lower with RUX versus vehicle; none were clinically significant.
For full article follow the link: DOI: 10.1016/j.jaad.2021.04.085
Primary source: Journal of the American Academy of Dermatology
Dr Satabdi Saha (BDS, MDS) is a practicing pediatric dentist with a keen interest in new medical researches and updates. She has completed her BDS from North Bengal Dental College ,Darjeeling. Then she went on to secure an ALL INDIA NEET PG rank and completed her MDS from the first dental college in the country – Dr R. Ahmed Dental College and Hospital. She is currently attached to The Marwari Relief Society Hospital as a consultant along with private practice of 2 years. She has published scientific papers in national and international journals. Her strong passion of sharing knowledge with the medical fraternity has motivated her to be a part of Medical Dialogues.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


