- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Spesolimab beneficial for prevention of generalized pustular psoriasis flares: Effisayil 2 trial
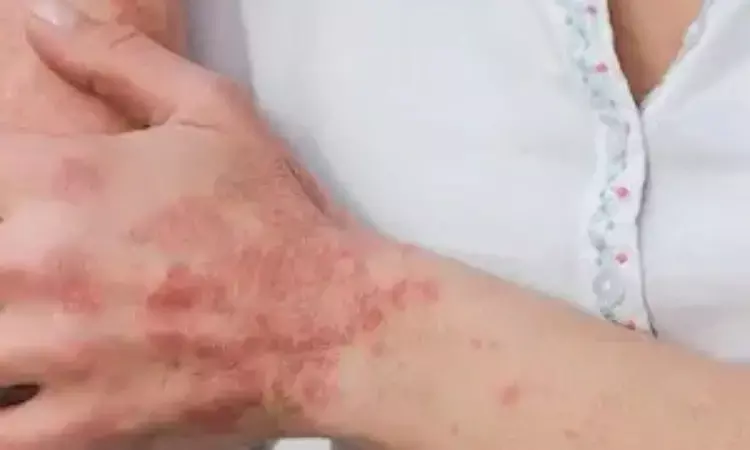
USA: New late-breaking data from the Effisayil 2 trial revealed an 84% reduction in the risk for generalized pustular psoriasis (GPP) flares with Spesolimab over the course of 48 weeks compared to placebo.
Additionally, no flares were observed after week 4 of spesolimab treatment in the high-dose group among 123 trial participants.
The pharmaceutical company Boehringer Ingelheim presented findings from the EFFISAYIL 2 trial, the largest and the first multinational, randomized clinical trial evaluating treatments for the prevention of GPP flares, at the 25th World Congress of Dermatology (WCD).
“These results provide further compelling clinical evidence for the role IL-36 signalling plays in the GPP pathogenesis,” Bruce Strober, MD, PhD, Clinical Professor, Dermatology at Yale University, said in a statement. “Moving forward, we hope that dermatologists not only have a specific treatment for GPP flares but that we can effectively prevent them in the future.”
Flares in GPP patients are characterized by papules on the body, which are known to be painful and frequently lead to emergency care, in addition to causing near-fatal complications such as organ failure, sepsis, and shock. The uncertainty GPP patients live with regarding the possible effects of their next flare that might occur can emotionally burden patients in a substantial way.
Spesolimab, an approved GPP flare treatment, is a novel, humanized, selective antibody known to block the activation of the interleukin-36 receptor (IL-36R). This signalling pathway in the body’s immune system is involved in autoinflammatory disease pathogenesis.
"Through our comprehensive EFFISAYIL clinical program, we have already delivered spesolimab as a major advancement for flaring GPP patients," Carinne Brouillon, Head of Human Pharma at Boehringer Ingelheim, said in a statement. "The EFFISAYIL 2 trial results build on this success bringing us closer to achieving our ultimate goal of a flare-free future for everyone living with GPP."
The phase 2 trial, EFFISAYIL 1, revealed that administering a solitary intravenous infusion of spesolimab led to swift alleviation of pustular and skin symptoms in those experiencing flares.
These findings were essential in obtaining regulatory approval for spesolimab, which became the pioneering targeted therapy for managing such flares in adult patients.
In the EFFISAYIL 2 trial, spesolimab was shown to have a favourable safety profile, with a similar incidence of patients with adverse events across spesolimab and placebo treatment arms.
Spesolimab has recently received Breakthrough Therapy Designation (BTD) from the U.S. FDA (Food and Drug Administration) and the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) as an investigational treatment for the prevention of GPP flares.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


