- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tapinarof cream effective for patients with plaque psoriasis: Study
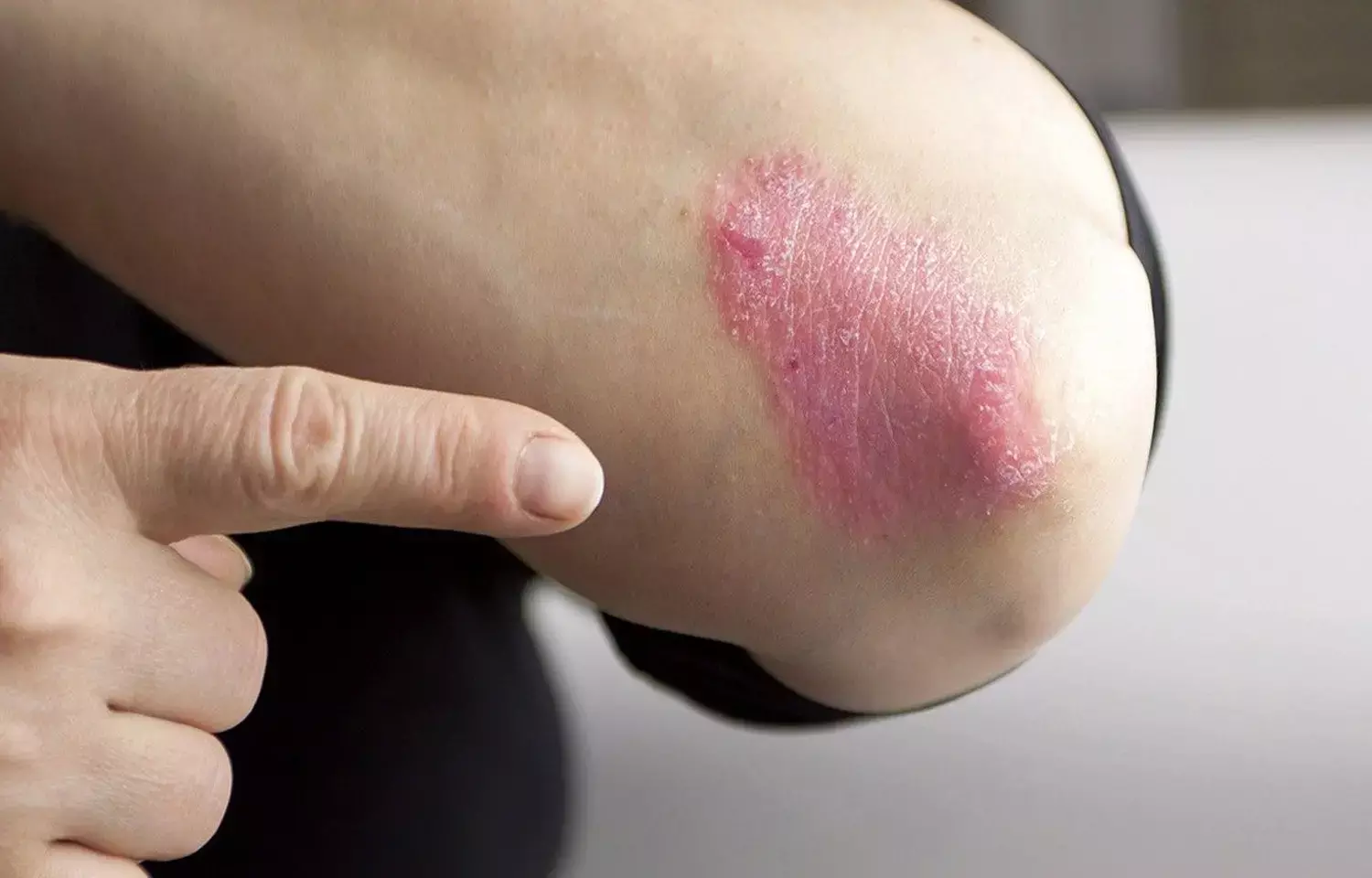
USA: Findings from two identical phase 3 trials showed the once-daily application of tapinarof 1% cream to be superior to vehicle control in reducing plaque psoriasis severity over a period of 12 weeks. However, the cream was associated with local adverse events and headaches. The study appears in the New England Journal of Medicine.
The authors stress the need for larger and longer trials for evaluating the safety and efficacy of tapinarof cream versus existing treatments for psoriasis.
Tapinarof cream is a topical aryl hydrocarbon receptor–modulating agent under investigation for psoriasis treatment. Tapinarof modulates the expression of interleukin-17 and the skin-barrier proteins filaggrin and loricrin. Mark G. Lebwoh and the team conducted two identical phase 3 randomized trials of tapinarof in patients with mild-to-severe plaque psoriasis.
It included adults with a baseline Physician's Global Assessment (PGA) score of 2 (mild) to 4 (severe) (on a scale from 0 to 4, with higher scores indicating more severe psoriasis) and a percent of total body surface area affected of 3 to 20%. They were randomly assigned in a ratio of 2:1 to receive tapinarof 1% cream or vehicle cream once daily for 12 weeks.
The primary endpoint was PGA response -- a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points at week 12.
Patient-reported outcomes were the mean changes from baseline to week 12 in the proportion of patients who had a decrease of at least 4 points in the Peak Pruritus Numeric Rating Scale (PP-NRS) score (range, 0 [no itch] to 10 [worst imaginable itch]), the PP-NRS total score, the Dermatology Life Quality Index total score, and the Psoriasis Symptom Diary score.
In trials 1 and 2, a total of 692 and 674 patients, respectively, were screened, with 510 and 515 patients being enrolled.
Following were the study's key findings:
- A PGA response occurred in 35.4% of the patients in the tapinarof group and in 6.0% of those in the vehicle group in trial 1 and in 40.2% and 6.3%, respectively, in trial 2.
- Results for secondary end points and patient-reported outcomes were generally in the same direction as those for the primary end point.
- Adverse events with tapinarof cream included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.
"Tapinarof 1% cream once daily was superior to vehicle control in reducing the severity of plaque psoriasis over a period of 12 weeks but was associated with local adverse events and headache," wrote the authors.
Reference:
The study titled, "Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis," is published in the New England Journal of Medicine.
DOI: https://www.nejm.org/doi/full/10.1056/NEJMoa2103629
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


