- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Teprotumumab- a novel treatment for pretibial myxedema
Source- 1. Mervis JS, Maeng MM, Kirsner RS, Wester ST. Teprotumumab as a novel treatment for pretibial myxedema. Br J Dermatol. 2022 Jun 6. doi: 10.1111/bjd.21700. Epub ahead of print. PMID: 35665912.
Teprotumumab- a novel treatment for pretibial myxedema
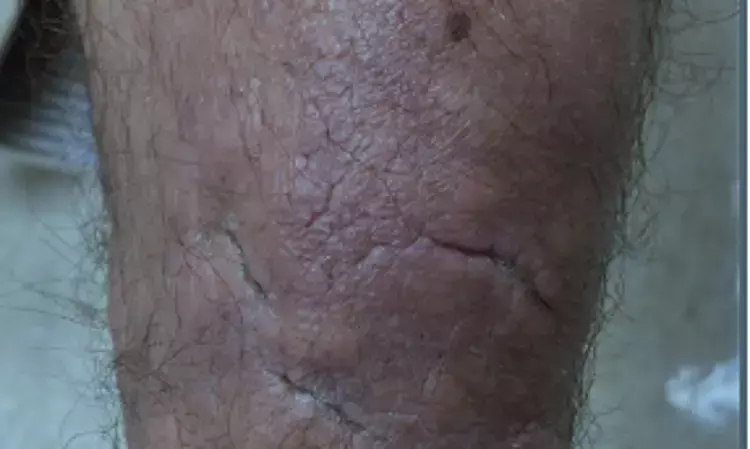
Pretibial myxedema (PTM) is an unusual extrathyroidal manifestation of autoimmune thyroid disease. It is mostly seen in association with thyroid eye disease (TED). PTM is characterized by an abnormal accumulation of glycosaminoglycans (mucin) in the dermis. Treatments tried in PTM hve not yielded good results so the search for an optimal therapy is still going on. Recently a case report displaying efficacy of teprotumumab in PTM was published in the British Journal of Dermatology.
Clinical forms of PTM range from diffuse nonpitting edema of the lower extremities to indurated, waxy, hyperpigmented to orange-red plaques and nodules, often with a peau d'orange texture. Extensive skin lesions can be extremely disfiguring and can cause severe physical impairment. Topical and intralesional steroids are often not effective, while systemic treatments including intravenous immunoglobulin (IVIg) and rituximab have been tried with variable degrees of success.
Teprotumumab is an insulin-like growth factor 1 receptor (IGF-1R) inhibitor approved by the US-FDA in January 2020 for TED and administered as a series of eight infusions, initially 10 mg/kg followed by 20 mg/kg every three weeks for seven additional doses. IGF-1R colocalizes with thyroid-stimulating hormone receptor (TSHR) in human orbital fibroblasts, forming a signalling complex. Patients with Graves' disease have IGF-1R activating autoantibodies capable of inducing hyaluronic acid production by orbital fibroblasts.
Despite the suspected shared pathophysiology of TED and PTM, the effect of teprotumumab on PTM has not been formally studied. The authors retrospectively assessed response to treatment in a cohort of patients with PTM who received teprotumumab for TED. Patients treated with at least one infusion of teprotumumab between January 2020 and June 2021 were interviewed via telephone and their medical records were retrospectively reviewed. Patients in whom teprotumumab infusions were ongoing or discontinued prior to completion of treatment were included.
Twenty-seven patients with TED received teprotumumab during the study period, of which 23 consented to the telephone survey. Ten of 23 respondents reported a history of skin changes consistent with PTM. Four of 10 had previously been diagnosed with PTM by a dermatologist. Two had completed all eight infusions, one had completed four infusions, and one had completed three infusions at the time of interview. All four patients reported at least 50% improvement in PTM, with three reporting complete or near complete (>90%) resolution. None were using concurrent treatments for PTM or TED.
Amongst the additional six patients, 4 of the 6 reported at least 50% improvement in lower extremity skin changes after treatment with teprotumumab, including two who reported complete or near complete (>90%) resolution. Durability data for teprotumumab in TED is emerging, but it appears that some patients may need retreatment. Common adverse events include hyperglycemia, hearing loss, muscle spasms, alopecia, diarrhea, nausea, and fatigue.
In conclusion, PTM is a potentially debilitating disease and teprotumumab may be a new effective treatment for this condition.
References
- Mervis JS, Maeng MM, Kirsner RS, Wester ST. Teprotumumab as a novel treatment for pretibial myxedema. Br J Dermatol. 2022 Jun 6. doi: 10.1111/bjd.21700. Epub ahead of print. PMID: 35665912.
MBBS
Dr Manoj Kumar Nayak has completed his M.B.B.S. from the prestigious institute Bangalore medical college and research institute, Bengaluru. He completed his M.D. Dermatology from AIIMS Rishikesh. He is actively involved in the field of dermatology with special interests in vitiligo, immunobullous disorders, psoriasis and procedural dermatology. His continued interest in academics and recent developments serves as an inspiration to work with medical dialogues.He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


