- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tralokinumab and Topical Corticosteroids tied to sustained important in Atopic Dermatitis: ECZTRA 3 trial.
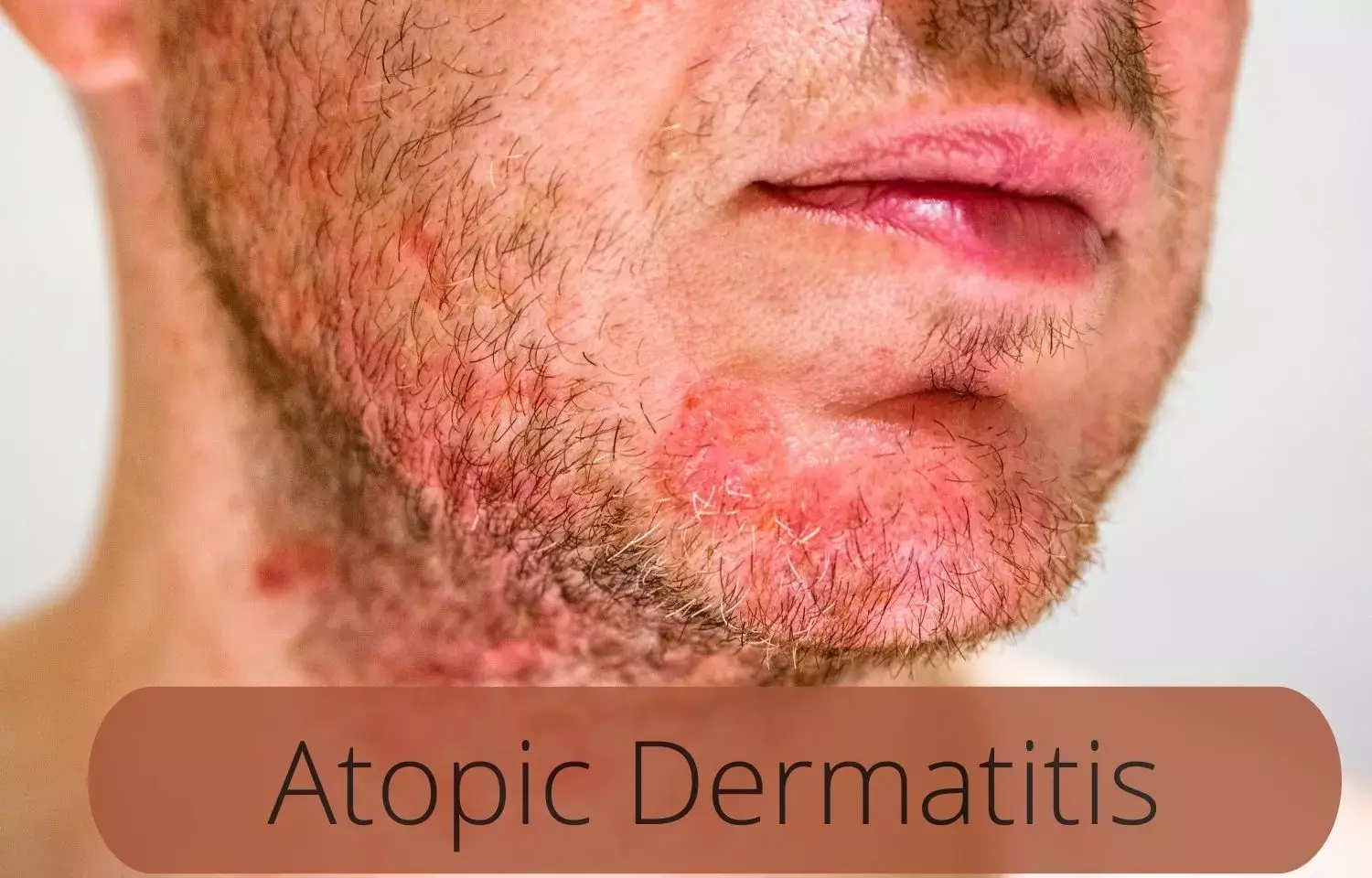
Over 32 weeks, it was found that continued use of tralokinumab treatment plus topical corticosteroids as needed provided progressive and sustained improvements in Atopic Dermatitis signs, symptoms, and health-related Quality of life as published in the journal, "American Journal of Clinical Dermatology, 2022."
Atopic dermatitis (AD) is a chronic inflammatory skin disease having a complex etiology leading to abnormalities in the epidermis and the immune system. Tralokinumab is a fully human monoclonal antibody that specifically neutralizes interleukin-13. The phase III ECZTRA 3 trial studied the efficacy and safety of tralokinumab with topical corticosteroids (TCS) as needed over 32 weeks. At Week 16, the primary endpoints of Investigator's Global Assessment (IGA) score of 0/1 and 75% improvement in Eczema Area and Severity Index (EASI-75) and all confirmatory endpoints were significantly achieved by tralokinumab- versus placebo-treated patients. So, researchers conducted a post hoc analysis to investigate the impact of tralokinumab plus TCS on AD severity, symptoms, and health-related quality of life (QoL) over the entire 32-week treatment period of ECZTRA 3, including all patients initiated on tralokinumab irrespective of the response achieved at Week 16.
Randomizing the participants to 2:1, to receive subcutaneous tralokinumab 300 mg or placebo every 2 weeks (q2w) with TCS as needed for an initial 16 weeks. At 16 weeks, patients who achieved the clinical response criteria were re-randomized 1:1 to tralokinumab q2w or every 4 weeks (q4w), with TCS as needed, for another 16 weeks. If they did not achieve the clinical response criteria, they were given tralokinumab q2w plus TCS from Week 16. All the participants randomized to tralokinumab in the initial treatment period were pooled for this analysis, irrespective of response.
Results
Continued tralokinumab (q2w, N = 164; q4w, N = 69) plus TCS treatment provided progressive improvements from Week 16 onwards in AD signs, with 70.2% of patients achieving EASI-75 and 50.4% achieving EASI-90 at Week 32.
Within the first few weeks of tralokinumab, q2w plus TCS treatment improvements in patient-reported outcomes were observed and were sustained throughout the 32 weeks.
At Week 32, patients initiated on tralokinumab q2w plus TCS achieved a relative improvement versus a baseline of 70.8% on the eczema-related sleep interference numeric rating scale (NRS) and 66.8% on Dermatology Life Quality Index (DLQI).
Mean TCS use during Weeks 16–32 ranged from 9.2 to 13.6 g q2w.
Most patients initiated on tralokinumab q2w plus TCS achieved a meaningful improvement in at least one of the three disease domains, including AD signs (EASI-50), symptoms (pruritus NRS improvement ≥ 3), and QoL (DLQI improvement ≥ 4) at Week 16.
Of patients initiated on tralokinumab q2w plus TCS, 53.4% achieved a clinically meaningful improvement in all three domains at Week 16.
Thus, the researchers concluded that over 32 weeks, continued tralokinumab treatment plus TCS, as needed, provides progressive and sustained improvements in AD signs, symptoms, and health-related QoL.
To read the full article, click here: https://doi.org/10.1007/s40257-022-00702-2
Silverberg JI, Adam DN, Zirwas M, et al. Tralokinumab Plus Topical Corticosteroids as Needed Provides Progressive and Sustained Efficacy in Adults with Moderate-to-Severe Atopic Dermatitis Over a 32-Week Period: An ECZTRA 3 Post Hoc Analysis [published online ahead of print, 2022 Jul 20]. Am J Clin Dermatol. 2022;10.1007/s40257-022-00702-2.
BDS, MDS
Dr.Niharika Harsha B (BDS,MDS) completed her BDS from Govt Dental College, Hyderabad and MDS from Dr.NTR University of health sciences(Now Kaloji Rao University). She has 4 years of private dental practice and worked for 2 years as Consultant Oral Radiologist at a Dental Imaging Centre in Hyderabad. She worked as Research Assistant and scientific writer in the development of Oral Anti cancer screening device with her seniors. She has a deep intriguing wish in writing highly engaging, captivating and informative medical content for a wider audience. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751



