- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
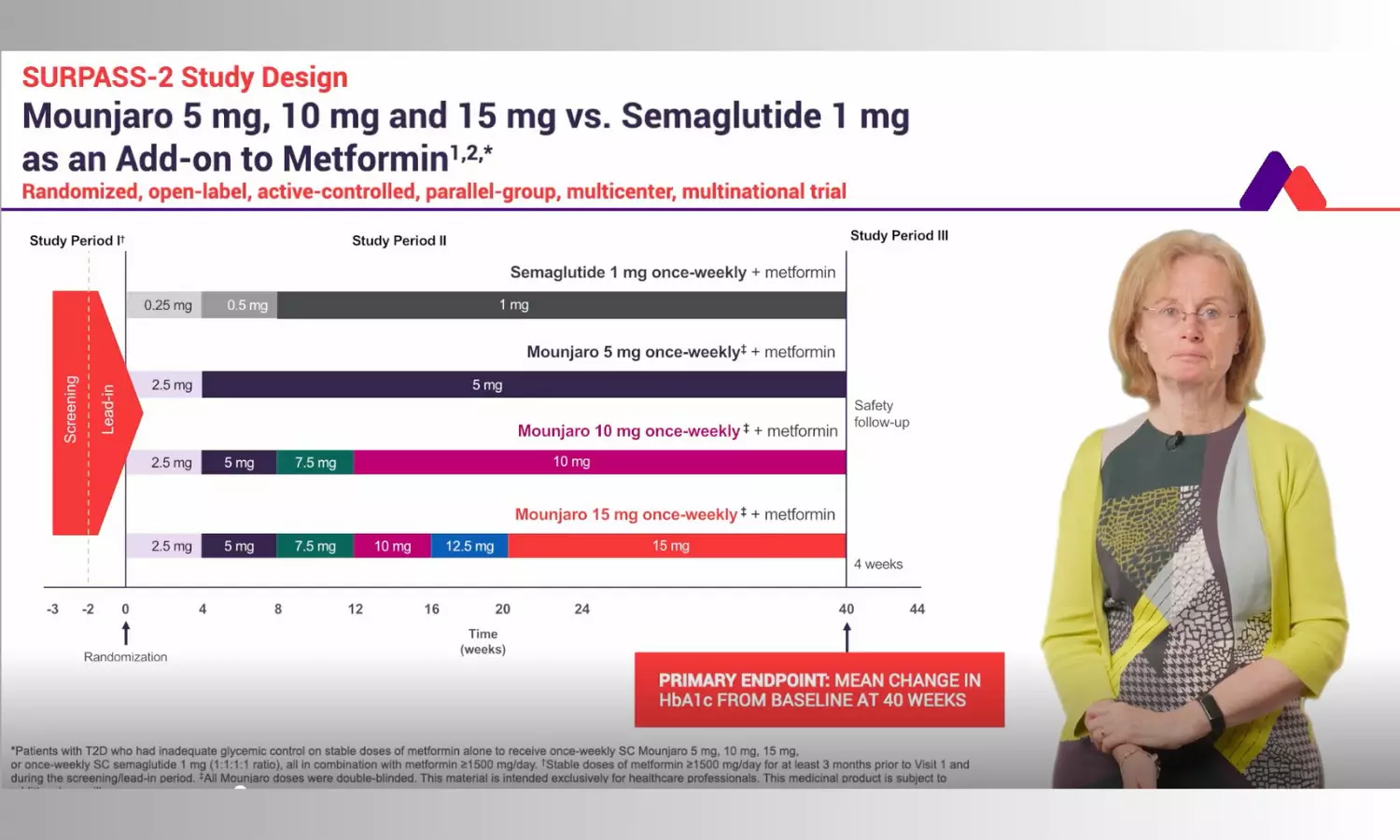
SURPASS-2: Superior HbA1c and Weight Reduction With Tirzepatide vs. Semaglutide - Video
Overview
The SURPASS-2 trial compared three doses of tirzepatide (5 mg, 10 mg, 15 mg) with semaglutide 1 mg, in adults with type 2 diabetes who were already taking metformin. The primary aim was to assess reduction in HbA1c levels. Tirzepatide produced greater HbA1c reductions than semaglutide, with decreases of 2.1% to 2.5% depending on dose, compared to 1.9% with semaglutide. 1,2 A significantly larger proportion of patients on the highest dose achieved tighter glycemic targets, including over 50% achieving HbA1c <5.7%, versus about 20% with semaglutide. 1,2,3
The trial also showed greater weight loss with tirzepatide. Patients on the 15 mg dose experienced an average weight loss of 12.4 kg, compared to 6.2 kg in those taking semaglutide. This demonstrates that tirzepatide not only improves glucose control but also offers a more substantial benefit in managing obesity, which is a major concern in type 2 diabetes. 1,2
In terms of safety, tirzepatide had a similar gastrointestinal side-effect profile to semaglutide, with nausea and other GI symptoms being the most common. Hypoglycemia rates were low across all treatment groups. 2 Overall, tirzepatide provided superior glycemic control and more pronounced weight reduction compared to semaglutide, while maintaining a comparable safety and tolerability profile. 2
THERAPEUTIC INDICATION1:
Type 2 diabetes mellitus
MOUNJARO® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
• as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
• in addition to other medicinal products for the treatment of diabetes.
For study results with respect to combinations, effects on glycaemic control and the populations studied, see sections 4.4, 4.5 and 5.1.
Weight management
MOUNJARO® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of
• ≥ 30 kg/m² (obesity) or
• ≥ 27 kg/m² to < 30 kg/m² (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes, or type 2 diabetes mellitus).
For Tirzepatide Prescribing Information, please check: https://image.mc.lilly.com/lib/fe9312747462077971/m/1/0e04e051-5fa2-445b-850d-4f09ef66d35e.pdf
Reference: 1. Tirzepatide India Prescribing Information | Updated March 2025. 2. Modified from Frias JP, et al, N Engl J Med. 2021;385(6): 503-515. 3. Perreault L. Prediabetes. In: Feingold KR, et al., eds. Endotext. MDText.com, Inc .; 2022. https://www.ncbi.nlm.nih.gov/books/NBK538537/(Accessed December 2023).
Disclaimer:
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. This material is being provided to healthcare professionals for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.
Mounjaro®, KwikPen® and Lilly are registered trademarks of Eli Lilly and Company. To be sold by retail under prescription of Endocrinologist or Internal Medicine Specialists only. For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com. For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001 India Ph.: +91-124-4753000/01 | www.lilly.com/in.
PP-TR-IN-0948 | 28 January 2026
Eli Lilly and Company. All rights reserved.