- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tirzepatide for Obesity: Highlights from the SURMOUNT-1 Trial - Video
Overview
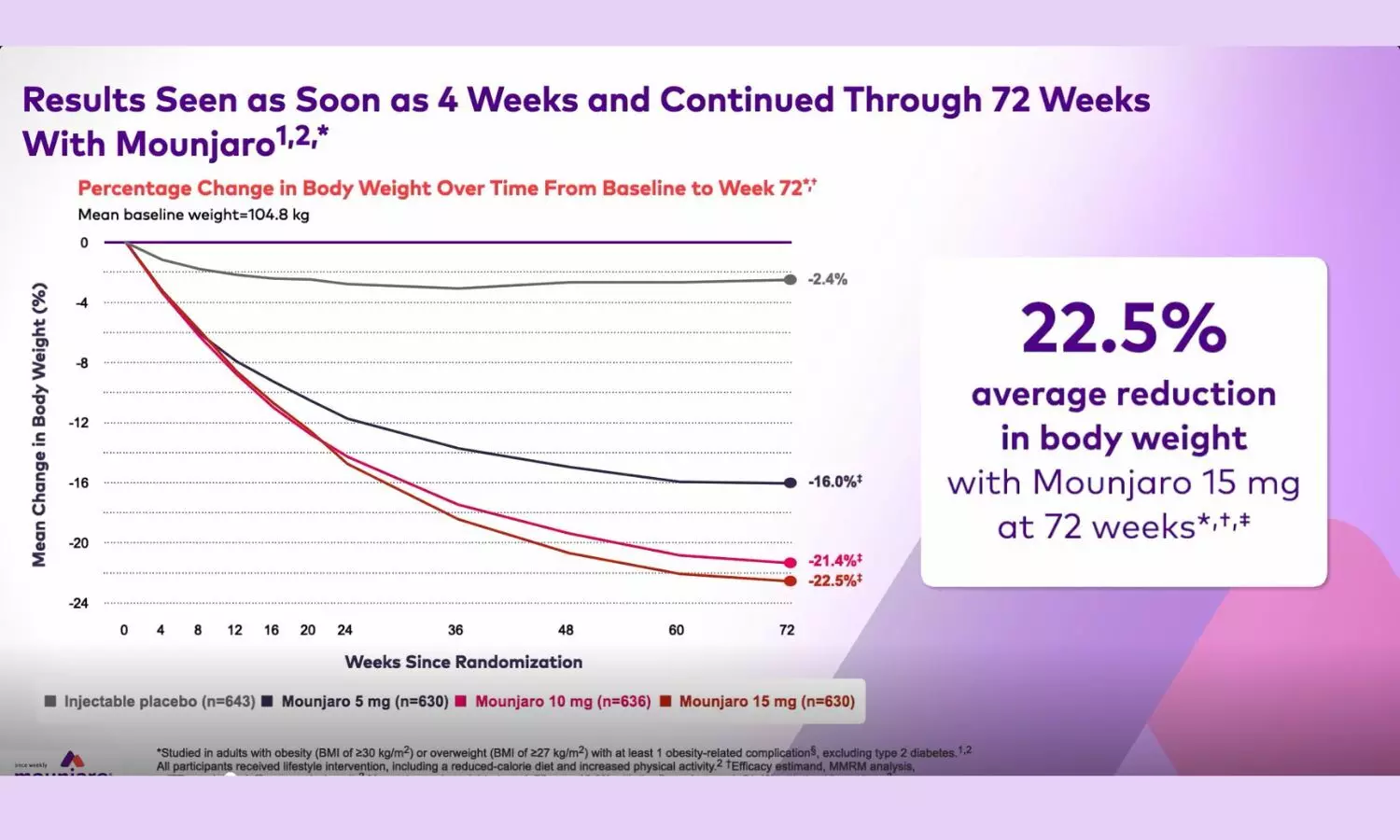
The SURMOUNT-1 trial evaluated Tirzepatide a dual GIP/GLP1 agonist, in individuals with overweight (BMI more than or equal to 27 with a comorbidity) or obesity (BMI more than or equal to 30) without type 2 diabetes.
The results indicate that, compared with placebo, Tirzepatide produced early and significant weight loss across all three doses (5 mg, 10 mg, and 15 mg). Weight reduction of approximately 16% was seen with the lowest dose of 5mg, and 21.4% to 22.5% with the doses of 10 and or 15 mg after the end of 72 weeks.
A categorical analysis revealed that nearly 63% of participants achieved ≥20% body weight loss, and about 40% (39.7%) achieved ≥25% weight loss.
Waist circumference reduced by ~20 cm with the highest dose. Most of the weight lost was fat mass (~34% reduction), with much smaller loss in lean mass, showing a favorable body composition effect.
Additionally, Tirzepatide improved several cardiometabolic parameters, including reductions in systolic/diastolic blood pressure and improvements in triglycerides, HDL, and LDL levels*.
Regarding safety, the side effect profile was consistent with other GLP-1 receptor agonists, primarily mild to moderate gastrointestinal symptoms (nausea, vomiting, diarrhea) most of these symptoms were seen during the early dose titration period and settled down over a period of time.
In conclusion, the SURMOUNT-1 trial demonstrates that Tirzepatide leads to substantial and clinically meaningful weight loss, improvement in cardiometabolic health, and is generally well tolerated, highlighting its therapeutic potential for individuals living with obesity.
*Results are not adjusted for multiplicity
THERAPEUTIC INDICATION1:
Type 2 diabetes mellitus
MOUNJARO® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
• as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
• in addition to other medicinal products for the treatment of diabetes.
Weight management
MOUNJARO® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of
• ≥ 30 kg/m² (obesity) or
• ≥ 27 kg/m² to < 30 kg/m² (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes, or type 2 diabetes mellitus).
For Tirzepatide Prescribing Information, please check: https://image.mc.lilly.com/lib/fe9312747462077971/m/1/0e04e051-5fa2-445b-850d-4f09ef66d35e.pdf
REFERENCES
1. Mounjaro® (tirzepatide), India Prescribing Information. Updated March 2025. 2. Jastrebo_ AM, et al. N Engl J Med. 2022;387(3):205–16.
Disclaimer:
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. The information provided in this section is intended solely for the use of registered medical practitioner. This material is being provided to healthcare professionals for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.
Mounjaro®, KwikPen® and Lilly are registered trademarks of Eli Lilly and Company. To be sold by retail under prescription of Endocrinologist or Internal Medicine Specialists only. For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com. For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001 India Ph.: +91-124-4753000/01 | www.lilly.com/in.
PP-TR-IN-0892 | 20 November 2025
Eli Lilly and Company. All rights reserved.