- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Remibrutinib oral therapy effective against Sjogren's syndrome
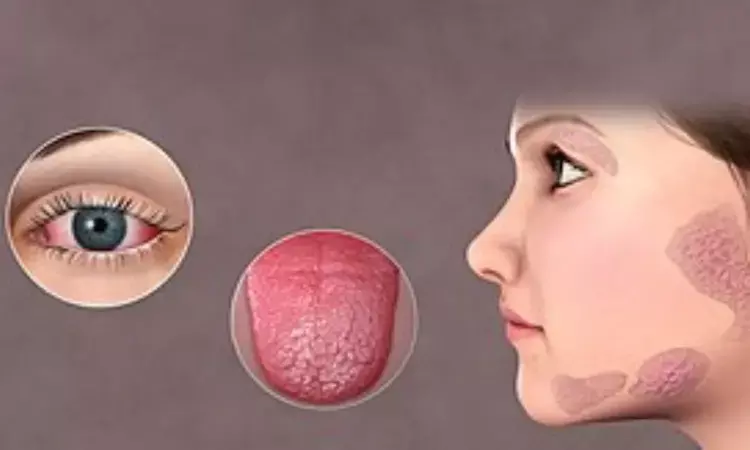
Over the course of 24 weeks, remibrutinib was well tolerated by Sjögren's syndrome (SS) patients and had a favorable safety profile. The findings were presented by Thomas Dörner in the American College of Rheumatology Convergence 2022.
Patients with SS are 15–20 times more likely to develop B-cell lymphoma, a potentially fatal consequence. Remibrutinib is an oral, covalent, and extremely effective Bruton's tyrosine kinase inhibitor (BTK). BTK is essential for B-cell receptor and Fc receptor signaling and is expressed in cells of the innate and adaptive immune systems, including B cells.
This randomized, double-blind, placebo-controlled phase 2 study recruited patients with moderate to severe Sjögren's syndrome (SS) who had a baseline EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) score of 5, an anti-Ro/SSA antibody positivity 3 months prior to screening, and an unstimulated whole salivary flow rate of >0 mL/min. The safety of remibrutinib in SS patients, as well as its influence on changes from baseline in ESSDAI, salivary flow, and patient reported outcomes (PRO), were investigated.
The key findings of this study were:
1. Between August 2019 and May 2021, 73 patients were randomly assigned to receive remibrutinib 100 mg bid (n=24), 100 mg qd (n=25), or placebo (n=24).
2. Only two of the patients were guys. The average (standard deviation) age was 51.8 (13.34) years.
3. In terms of demographic and disease-related baseline data, the groups were generally balanced.
4. The occurrence of adverse events (AEs) was comparable among groups. There were no significant adverse events recorded.
5. Infections were the most often reported adverse event by system organ class, with rates comparable in the remibrutinib and placebo groups.
6. There were no significant liver abnormalities detected in any of the groups.
7. At week 24, remibrutinib resulted in a significantly significant improvement in ESSDAI score compared to placebo, with numerical improvement over placebo at all time points.
8. When compared to placebo, unstimulated salivary flow improved with remibrutinib.
9. Total serum IgG and IgM levels fell from baseline but remained within acceptable limits. Autoantibodies against SS-A and SS-B followed the same pattern as total IgG.
10. PROs such as ESSPRI, Functional Assessment of Chronic Illness Therapy-Fatigue, and EuroQol-5 Dimension were comparable across the remibrutinib and placebo groups.
In conclusion, these findings point to remibrutinib as the first successful oral disease-modifying treatment for SS. PROs, like ESSPRI, may require a longer period of time to reveal advantages. More research is needed to validate the efficacy of remibrutinib therapy in SS.
Reference:
Dörner T, Szántó A, Tseng J, Kaul M, Pylvaenaeinen I, Hanser M, Abdallah N, Cenni B, Siegel R. Remibrutinib (LOU064) in Sjögren's Syndrome: Safety and Efficacy Results from a 24‑Week Placebo-controlled Proof-of-Concept Study [abstract]. ACR Convergence 2022; In American College of Rheumatology.
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


