- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Continued Treatment With Mirikizumab effective in Patients With Ulcerative Colitis: Study
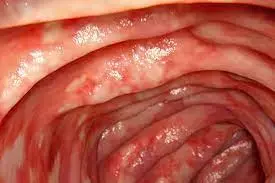
Extended doses (600 and 1000 mg) of mirikizumab effective in Phase 2 Trial of Patients with Ulcerative Colitis, according to a recent study published in the Clinical Gastroenterology and Hepatology.
Mirikizumab is an antibody against the p19 subunit of interleukin 23 that has demonstrated clinical efficacy and was well tolerated following 12 weeks of induction treatment in a phase 2 trial of patients with moderate to severe ulcerative colitis. We present results of the open-label extended induction period in patients who did not initially respond to treatment with mirikizumab.
This study was a continuation of I6T-MC-AMAC, a double-blind trial, performed at 75 sites in 14 countries, in which patients with moderate to severe ulcerative colitis were randomly assigned to 12 weeks of induction therapy with 50 mg, 200 mg, or 600 mg mirikizumab or placebo. Patients without a clinical response (a 9-point decrease in Mayo subscore of ≥2 points and ≥35% from baseline and either a decrease of rectal bleeding subscore of ≥1 or a rectal bleeding subscore of 0 or 1) at week 12 were offered the opportunity to participate in an open-label, extended induction study for another 12 weeks, in which they received either 600 mg intravenous mirikizumab (n = 20) or, following a protocol amendment, 1000 mg intravenous mirikizumab (n = 64) every 4 weeks. At week 24, patients with a clinical response continued the extended maintenance period and received 200 mg subcutaneous mirikizumab. Endpoints included clinical remission (Mayo subscores of 0 for rectal bleeding, 0 or 1 with a 1-point decrease from baseline), clinical response, endoscopic remission (Mayo endoscopic subscore of 0), or endoscopic improvement (endoscopic subscore of 0 or 1), at study weeks 24 and 52. Data were analysed for patients who received mirikizumab or placebo during the induction phase of the study.
The results of the study are as follows:
Among participants who did not respond to induction mirikizumab, 50.0% of those who received the 12-week extension of 600 mg mirikizumab and 43.8% who received the extension of 1000 mg mirikizumab achieved a clinical response; 15.0% and 9.4% achieved clinical remission, respectively. Endoscopic improvement was achieved by 20.0% of subjects in the 600 mg mirikizumab group and 15.6% of subjects in the 1000 mg mirikizumab group. Among initial nonresponders to mirikizumab who had a clinical response at study week 24 and continued into maintenance therapy, 65.8% maintained the clinical response, 26.3% achieved clinical remission, and 34.2% had endoscopic improvement at week 52. No new safety concerns were identified.
Thus, the researchers concluded that extended doses of mirikizumab (600 mg and 1000 mg) for an additional 12 weeks produce a clinical response in up to 50% of patients who did not have a clinical response to 12 weeks of induction doses (50 mg, 200 mg, or 600 mg). Most of the responders to the extended doses maintained clinical response for up to 52 weeks.
Reference:
Efficacy and Safety of Continued Treatment With Mirikizumab in a Phase 2 Trial of Patients With Ulcerative Colitis by William J. Sandborn et al. published in the Clinical Gastroenterology and Hepatology.
https://www.cghjournal.org/article/S1542-3565(20)31289-1/fulltext
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


