- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA Approves Updated mRNA Vaccines to Combat Emerging COVID-19 Variants
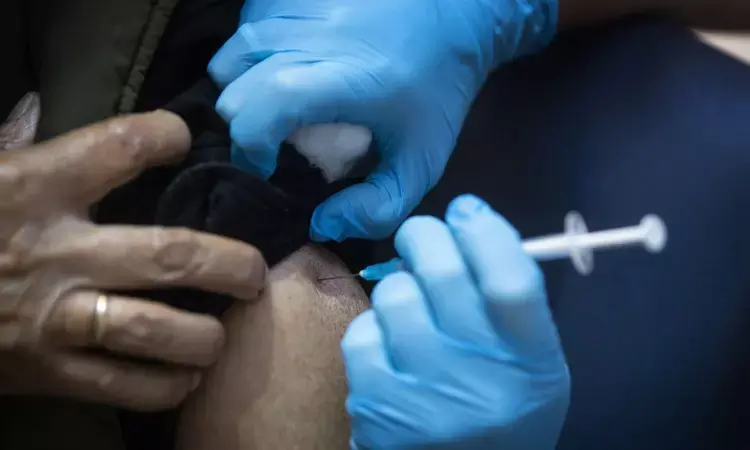
The US Food and Drug Administration (FDA) has officially granted emergency use authorization for updated COVID-19 vaccines that are designed to better target the currently circulating strains. This development sets the stage for a discussion tomorrow by the Centers for Disease Control and Prevention (CDC) vaccine advisory group regarding recommendations.
The FDA's approval covers both Moderna and Pfizer-BioNTech's mRNA vaccines. The FDA is still in the process of evaluating an updated vaccine from Novavax, which utilizes a more traditional cell-based manufacturing approach. This vaccine includes Novavax's proprietary matrix M adjuvant to enhance the body's immune response.
Novavax has stated that doses of its vaccine have arrived in the United States today and will be ready for distribution once FDA approval is granted. The company also intends to present the latest data on its vaccine during tomorrow's meeting of the CDC's Advisory Committee on Immunization Practices (ACIP).
In June, following recommendations from its vaccine advisors, the FDA requested vaccine manufacturers to develop vaccines specifically targeting the XBB.1.5 Omicron variant. Since then, newer Omicron variants like EG.5 have been on the rise, along with a gradual increase in COVID-19 activity over the past 9 weeks. In August, all three vaccine manufacturers reported preliminary findings indicating that the updated vaccines stimulated an increase in neutralizing antibodies against newer variants such as EG.5, FL.1.5.1, and XBB.1.16.6. Around the same time, concerns emerged about the highly mutated BA.2.86 variant and its potential resistance to the new vaccines. The UK accelerated the rollout of these updated vaccines in late August as a precaution.
However, initial laboratory experiments suggest that the BA.2.86 variant may be less immune-evasive than initially feared. Moderna and Pfizer have recently reported promising responses against BA.2.86 in clinical and preclinical studies. The FDA's approval today extends eligibility for the updated mRNA vaccine to individuals aged 5 and older, regardless of their previous vaccination status. Specific recommendations for children aged 6 months to 4 years will be provided based on their prior immunization.
The FDA emphasizes its confidence in the safety and efficacy of these vaccines, with a risk-benefit analysis indicating that the benefits outweigh potential risks. The side-effect profile of the updated vaccines is similar to that of earlier mRNA vaccines. Dr. Peter Marks, who leads the FDA's Center for Biologics Evaluation and Research, underscores the critical role of vaccination in public health and protection against severe COVID-19 outcomes.
The CDC reports that BA.2.86 has been identified in patients from nine states, with wastewater detections in Ohio and New York. Health officials are also investigating one more human case. The agency states that its risk assessment remains unchanged since its August 30 update.
Reference:
FDA takes action on updated mRNA COVID-19 vaccines to better protect against currently circulating variants. (2023, September 11). U.S. Food and Drug Administration; FDA.
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


