- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Lebrikizumab effectively improves moderate to severe atopic dermatitis in phase 3 trial
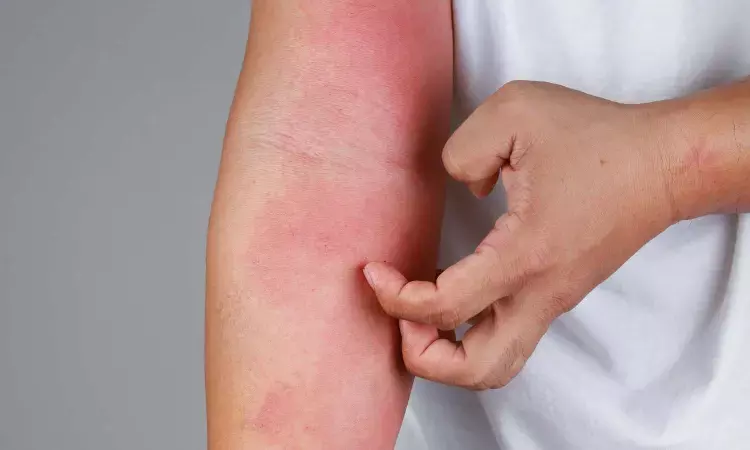
A new study published in The New England Journal of Medicine suggests that 16 weeks of lebrikizumab medication proved efficacious in adolescents and adults with mild-to-severe atopic dermatitis during the induction period of two phase 3 studies.
The interleukin-4R-interleukin-13R1 heterodimer receptor signaling complex is prevented by lebrikizumab, a high-affinity IgG4 monoclonal antibody targeting interleukin-13. Jonathan Silverberg and colleagues worked on this two-phase trial to investigate the effectiveness of Lebrikizumab on moderate to severe Atopic Dermatitis.
Researchers performed two 52-week, double-blind, randomized, placebo-controlled phase 3 studies, each with a 16-week induction phase and a 36-week maintenance term. Eligible patients with moderate-to-severe atopic dermatitis (adults [18 years old] and adolescents [12 to 18 years old, weighing 40 kg]) were randomly randomized in a 2:1 ratio to receive lebrikizumab at a dosage of 250 mg (loading dose of 500 mg at baseline and week 2) or placebo, delivered subcutaneously every 2 weeks. The induction period's outcomes were evaluated up to 16 weeks and have been incorporated into this study.
The primary goal was to achieve an Investigator's Global Assessment (IGA) score of 0 or 1 (meaning clear or nearly clear skin; range, 0 to 4 [severe illness]) with a decrease (showing improvement) of at least 2 points from baseline at week 16. Secondary objectives were a 75% recovery in the Eczema Area and Severity Index (EASI-75 response) and itch and itch interference with sleep evaluations. Safety was also evaluated.
The key findings of this study were:
In study 1, 43.1% of 283 patients in the lebrikizumab group and 12.7% of 141 patients in the placebo group attained the main endpoint; an EASI-75 response occurred in 58.8% and 16.2%, respectively (P0.001).
The main endpoint in trial 2 was met in 33.2% of 281 patients in the lebrikizumab group and 10.8% of 146 patients in the placebo group (P0.001); an EASI-75 response occurred in 52.1% and 18.1%, respectively (P0.001).
Lebrikizumab medication improved itch and itch interference with sleep.
Conjunctivitis was more common in patients who got lebrikizumab than in those who received placebo.
The majority of adverse events throughout the induction phase were mild to moderate in severity and did not result in trial termination.
Reference:
Silverberg, J. I., Guttman-Yassky, E., Thaçi, D., Irvine, A. D., Stein Gold, L., Blauvelt, A., Simpson, E. L., Chu, C.-Y., Liu, Z., Gontijo Lima, R., Pillai, S. G., & Seneschal, J. (2023). Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. In New England Journal of Medicine (Vol. 388, Issue 12, pp. 1080–1091). Massachusetts Medical Society. https://doi.org/10.1056/nejmoa2206714
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


