- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Smallpox drug tecovirimat may reduce duration of symptoms and infectivity of monkeypox: Lancet
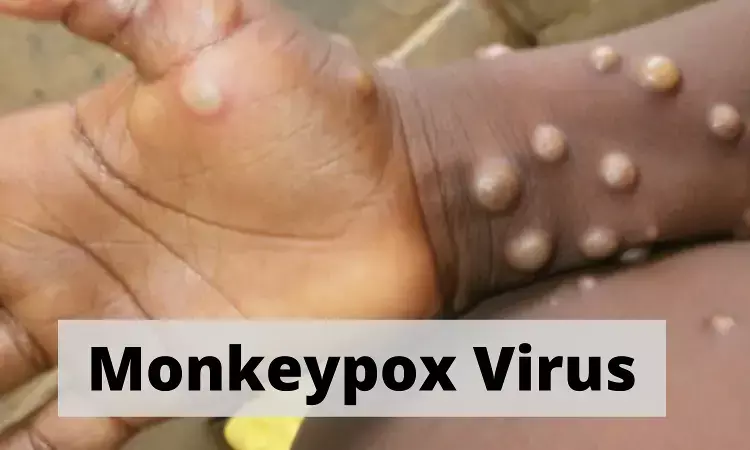
UK: The smallpox drug tecovirimat could reduce symptoms and contagiousness, a study on antivirals in seven monkeypox patients has suggested. The study was published in The Lancet Infectious Diseases on May 24, 2022.
"It is the first report on in-hospital and household transmission of monkeypox outside of Africa," Hugh Adler, Liverpool School of Tropical Medicine, Liverpool, UK, and colleagues wrote in their study.
Human monkeypox is a zoonosis whose cause is monkeypox virus. It was reported first in central Africa in 1970 and has affected some of the poorest and most marginalised communities in the world. The clinical syndrome is characterised by rash, fever, and lymphadenopathy.Its complications include encephalitis, pneumonitis, secondary bacterial infections, and sight-threatening keratitis.
In the month of May, cases and outbreaks of the smallpox relative, typically confined to central and western Africa, have been reported in countries such as the United Kingdom, the United States, the United Arab Emirates, Slovenia, and the Czech Republic. The study did not include any cases from this most recent outbreak.
In the observational study, the National Health Service England High Consequence Infectious Diseases (Airborne) Network retrospectively studied patient charts for virologic findings and response to the off-label antivirals tecovirimat and brincidofovir in four men and three women diagnosed as having monkeypox in Liverpool and Newcastle, England, from Aug 15, 2018, to Sep 10, 2021.
Three patients acquired monkeypox in the United Kingdom, including a healthcare worker infected at work and a child and adult who were household contacts of a person infected in West Africa. All patients, who were young and had no underlying illnesses, were hospitalized owing to the risk of viral transmission rather than to the severity of disease.
Signs and symptoms include the presence of virus in the blood, low mood likely related to isolation or perceived stigma, in one patient, a deep-tissue abscess that tested positive for the virus on polymerase chain reaction (PCR) testing, and prolonged viral DNA in the upper respiratory tract. Five patients with prolonged PCR-positivity after the crusting of all lesions were isolated for 22 to 39 days.
Treatment had to be stopped in three patients treated with 200 milligrams (mg) oral once-weekly brincidofovir following the development of elevated liver enzymes, indicating liver inflammation or injury.
The patient given 200 mg of oral tecovirimat twice daily for 2 weeks had no adverse effects and shed viral DNA for only 10 days. The authors wrote, "The patient treated with tecovirimat had a shorter duration of symptoms and upper respiratory tract viral shedding than the other patients in the series."
One patient had a mild resurgence of monkeypox 6 weeks after hospital release. All patients had mild illnesses and full recoveries.
The authors cautioned that the sample size was very small but said the need for monkeypox treatments is urgent.
"Although optimum infection control and treatment strategies for this potentially dangerous pathogen are not established, our first-use data suggest brincidofovir has poor efficacy; however, prospective studies of tecovirimat in human monkeypox are warranted," they wrote.
"Monkeypox outbreaks will continue to occur in west and central Africa, and health-care workers around the world must remain vigilant to the possibility of monkeypox in travellers presenting with fever and rash," the researchers added.
In a Lancet news release, senior author Nicholas Price, PhD, of Guy's & St. Thomas' NHS Foundation Trust, said that the study and the recent monkeypox outbreaks underscore the importance of maintaining a network of centers to manage sporadic outbreaks of high-consequence diseases such as monkeypox.
"The cases we observed were challenging and resource-intensive to manage, even in the high-income setting of the UK," he wrote. "With international travel returning to pre-pandemic levels, public health officials and healthcare workers around the world must remain vigilant to the possibility of new cases of monkeypox."
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


