- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tacrolimus an alternative to IV cyclophosphamide as initial therapy for Lupus Nephritis
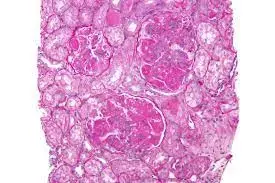
Tacrolimus is non-inferior to intravenous cyclophosphamide (IVCY) and is a good alternative for initial therapy of active Lupus Nephritis (LN), according to a recent study published in the JAMA Network Open.
Lupus nephritis (LN) is typically treated with intravenous cyclophosphamide (IVCY), which is associated with serious adverse effects. Tacrolimus may be an alternative for the initial treatment of Lupus Nephritis (LN); however, large-scale, randomized clinical studies of tacrolimus are lacking.
A study was conducted to assess the efficacy and safety of tacrolimus vs intravenous cyclophosphamide (IVCY) as initial therapy for Lupus Nephritis (LN) in China.
This randomized (1:1), open-label, parallel-controlled, phase 3, noninferiority clinical trial recruited patients aged 18 to 60 years with systemic lupus erythematosus and LN class III, IV, V, III+V, or IV+V primarily from outpatient settings at 35 centres in China. Inclusion criteria included body mass index of 18.5 or greater to less than 27, 24-hour urine protein of 1.5 g or greater, and serum creatinine of less than 260 μmol/L. Of 505 patients screened, 191 failed screening (163 ineligible, 25 withdrawn consents, and 3 other reasons). Overall, 314 were randomized. The first patient was enrolled on March 10, 2015, and the study finished on September 13, 2018. The follow-up period was 24 weeks. Data were analyzed from December 2019 to March 2020.
The results of the study are:
A total of 314 patients were randomized. Overall, 299 patients (95.2%) were treated. Baseline demographic and clinical characteristics were generally similar between groups (mean [SD] age, 34.2 [9.5] years; 262 [87.6%] female). Tacrolimus was found to be non-inferior to intravenous cyclophosphamide (IVCY) for Lupus Nephritis (LN) response at week 24. There was a complete or partial response rate of 83.0% (117 of 141 patients) in the tacrolimus group and 75.0% (93 of 124 patients) in the intravenous cyclophosphamide (IVCY) group (difference, 7.1%; 2-sided 95% CI, −2.7% to 16.9%; lower limit of 95% CI greater than −15%). At week 24, the least-square mean change in Systemic Lupus Erythematosus Disease Activity Index score was −8.6 with tacrolimus and −6.4 with intravenous cyclophosphamide (IVCY) (difference, −2.2; 95% CI, −3.1 to −1.3). Changes in other immune parameters and kidney function were generally similar between groups. Serious treatment-emergent adverse events (TEAEs) were reported in 29 patients in the tacrolimus group (18.5%) and 35 patients in the intravenous cyclophosphamide (IVCY) group (24.6%). The most common serious study drug-related treatment-emergent adverse events (TEAEs) were infections (14 [8.9%] and 23 [16.2%], respectively). Seven patients in each group withdrew due to treatment-emergent adverse events (TEAEs)
Thus, researchers concluded that in this study, oral tacrolimus appeared noninferior to intravenous cyclophosphamide (IVCY) for initial therapy of active Lupus Nephritis (LN), with a more favourable safety profile than intravenous cyclophosphamide (IVCY). Tacrolimus may be an alternative to intravenous cyclophosphamide (IVCY) as initial therapy for Lupus Nephritis (LN).
Reference:
Effect of Tacrolimus vs Intravenous Cyclophosphamide on Complete or Partial Response in Patients with Lupus Nephritis: A Randomized Clinical Trial by Zhaohui Zheng, et al. published in the JAMA Network Open.
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


