- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Cognitive Worsening and Absence of Benefit in HSV-Seropositive Early Alzheimer Disease: JAMA
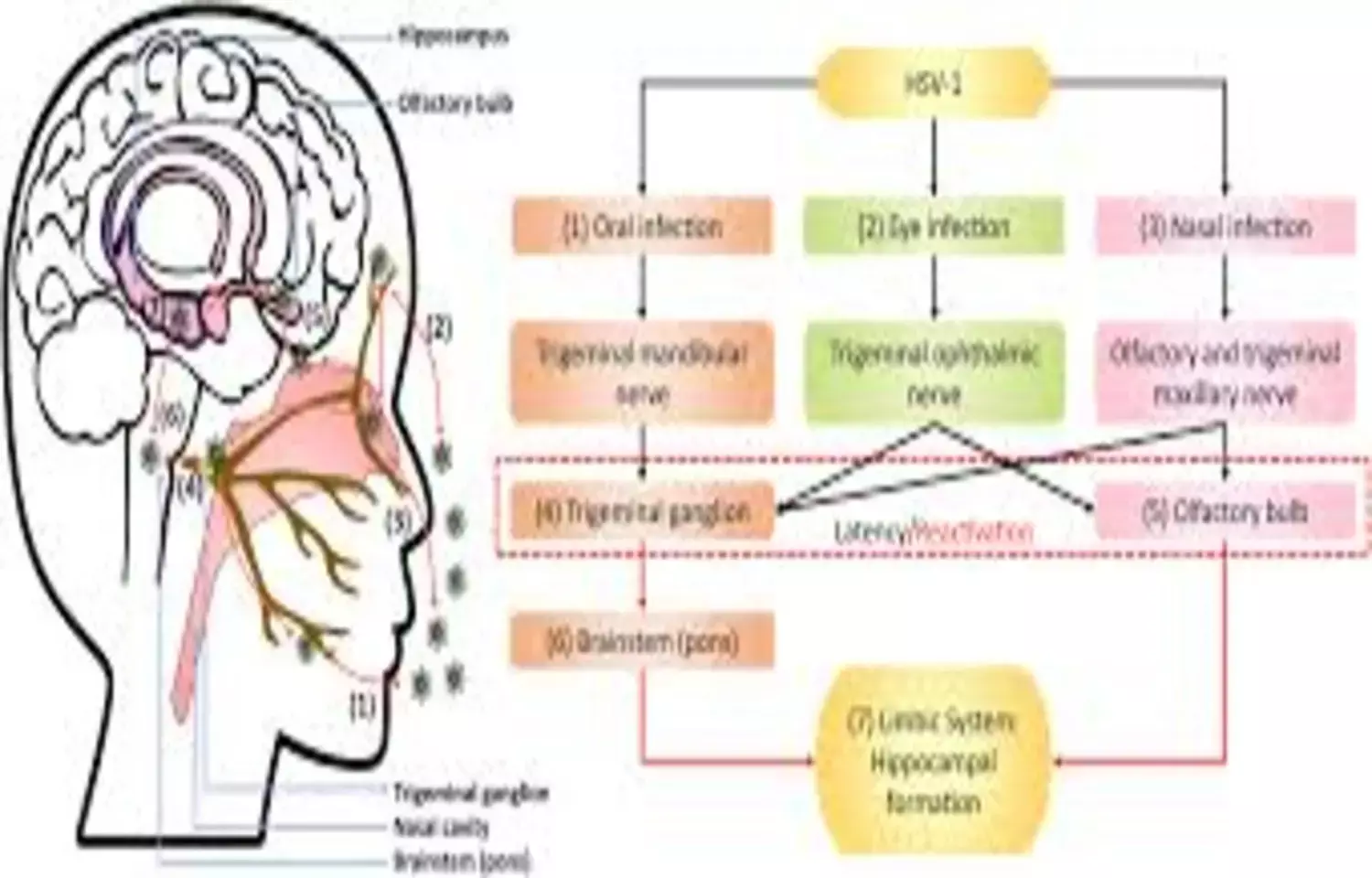
A new study published in JAMA has revealed that Valacyclovir did not improve cognitive outcomes and was associated with cognitive worsening in the primary outcome, indicating that it is not recommended for treating individuals with early symptomatic Alzheimer disease who are herpes simplex virus–seropositive.
Neuroscientific, epidemiological, and electronic health record studies implicate herpes simplex virus (HSV) as potentially etiological for Alzheimer disease (AD). A study was done to compare the efficacy and adverse effects of valacyclovir vs placebo in participants with early symptomatic AD and HSV seropositivity (HSV-1 or HSV-2). This randomized clinical trial included adults with a clinical diagnosis of probable AD or a clinical diagnosis of mild cognitive impairment with positive biomarkers for AD, a positive serum antibody test (IgG or IgM) for HSV-1 or HSV-2, and a Mini-Mental State Examination score of 18 to 28. The trial was conducted at 3 US outpatient clinics specializing in memory disorders. Recruitment occurred from January 2018 to May 2022; the last follow-up occurred in September 2024. The primary outcome was least-squares mean (LSM) change at 78 weeks in the 11-item Alzheimer’s Disease Assessment Scale Cognitive (ADAS-Cognitive) Subscale score (range, 0-70; higher scores indicate greater impairment). The secondary outcomes were LSM change in the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) Scale score; LSM change in the 18F-florbetapir amyloid positron emission tomography (PET) standardized uptake value ratio (SUVR; higher scores indicate higher amyloid levels) for 6 brain regions (medial orbitofrontal, anterior cingulate, parietal lobe, posterior cingulate, temporal lobe, and precuneus); and LSM change in 18F-MK-6240 tau PET medial temporal SUVR (higher scores indicate higher tau levels) for 4 brain regions (amygdala, hippocampus, entorhinal, and parahippocampus). The frequency of adverse events was the safety outcome.
Results Of the 120 participants (mean age, 71.4 [SD, 8.6] years; 55% were female), 93 (77.5%) completed the trial. At 78 weeks, the LSM change in the 11-item ADAS-Cognitive Subscale score was 10.86 (95% CI, 8.80 to 12.91) in the valacyclovir group vs 6.92 (95% CI, 4.88 to 8.97) in the placebo group, indicating greater cognitive worsening with valacyclovir than placebo (between-group difference, 3.93 [95% CI, 1.03 to 6.83]; P = .01). The LSM change in the ADCS-ADL Scale score at 78 weeks was −13.78 (95% CI, −17.00 to −10.56) in the valacyclovir group vs −10.16 (95% CI, −13.37 to −6.96) in the placebo group (between-group difference, −3.62 [95% CI, −8.16 to 0.93]). At 78 weeks, the LSM change in the 18F-florbetapir amyloid PET SUVR was 0.03 (95% CI, −0.04 to 0.10) in the valacyclovir group vs 0.01 (95% CI, −0.06 to 0.08) in the placebo group (between-group difference, 0.02 [95% CI, −0.08 to 0.12]). The LSM change in the 18F-MK-6240 tau PET medial temporal SUVR at 78 weeks was 0.07 (95% CI, −0.06 to 0.19) in the valacyclovir group vs −0.04 (95% CI, −0.15 to 0.07) in the placebo group (between-group difference, 0.11 [95% CI, −0.06 to 0.28]). The most common adverse events were elevated serum creatinine level (5 participants [8.3%] in the valacyclovir group vs 2 participants [3.3%] in the placebo group) and COVID-19 infection (3 [5%] vs 2 [3.3%], respectively).
Valacyclovir was not efficacious with cognitive worsening for the primary outcome and it is not recommended to treat individuals with early symptomatic AD and HSV seropositivity.
Devanand DP, Wisniewski T, Razlighi Q, et al. Valacyclovir Treatment of Early Symptomatic Alzheimer Disease: The VALAD Randomized Clinical Trial. JAMA. 2026;335(6):511–522. doi:10.1001/jama.2025.21738
Keywords:
Cognitive, Worsening, Absence, Benefit, HSV-Seropositive, Early, Alzheimer, Disease, JAMA, Devanand DP, Wisniewski T, Razlighi
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.


