- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA Clears novel Neurostimulation Device to treat Diabetic Neuropathic Pain
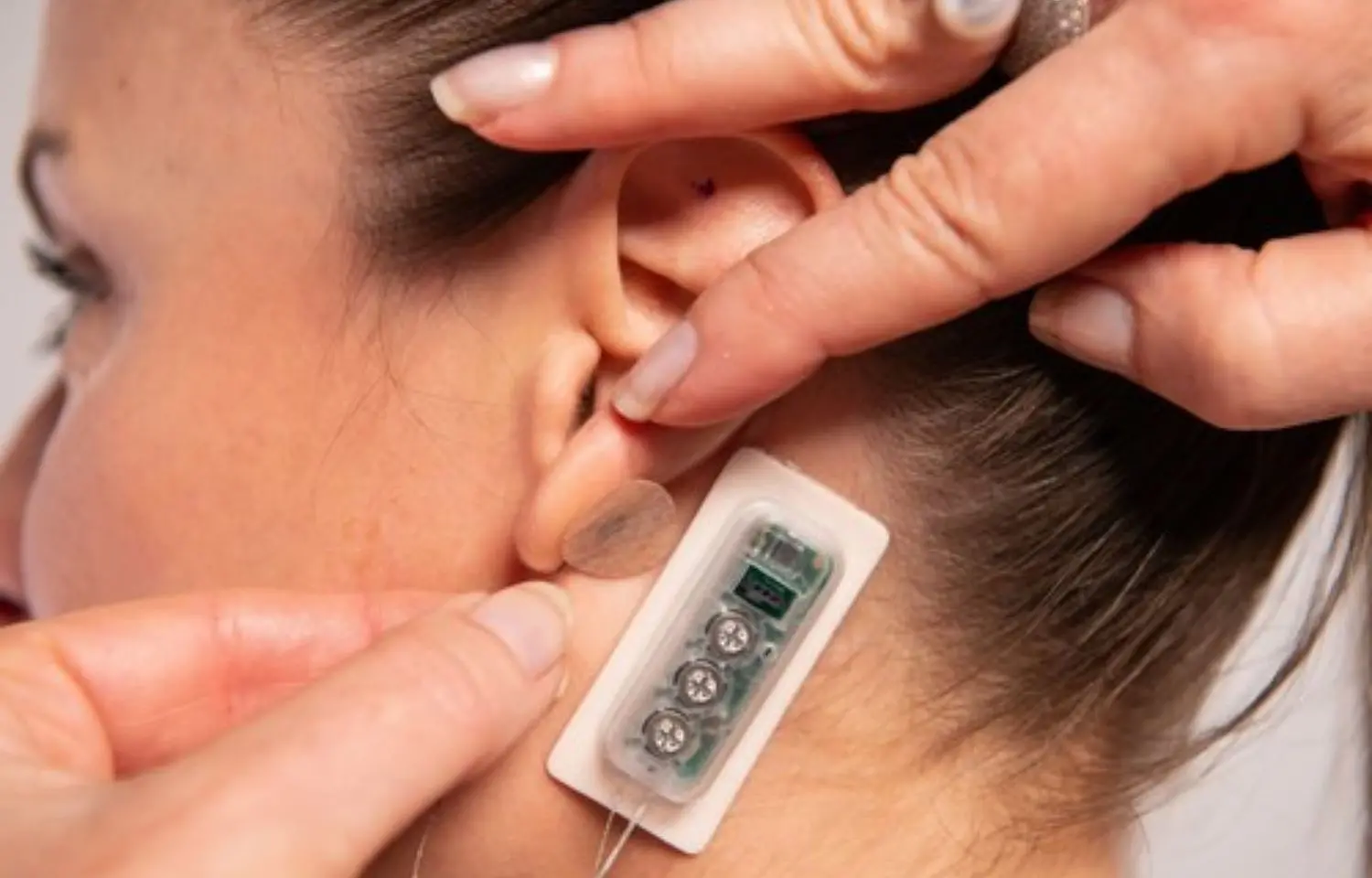
The US Food and Drug Administration has cleared a percutaneous electrical neurostimulation (PENS) device for symptomatic relief of chronic, intractable pain from diabetic peripheral neuropathy.
"We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy, said DyAnsys CEO Srini Nageshwar. "First Relief offers a significant treatment option without drugs or narcotics."
The approval was based on a study that tested First Relief against a placebo and another device previously cleared by the US Food and Drug Administration.
The study was conducted at the Jeevak Multispeciality Hospital in Warangal, India, renowned for the treatment of diabetes. The single center, three-arm, randomized, controlled, parallel assignment, double blinded, prospective study involved 63 patients age 30 to 74.
The devices were applied on a bi-weekly basis for 16 weeks. The primary efficacy endpoint was pain intensity measured through Visual Analog Scale (VAS) score and the secondary efficacy endpoints are vibration perception threshold (VPT) value, insomnia severity index (ISI), overall neuropathy limitations scale (ONLS), Hamilton rating scale for anxiety.
The VAS pain score analysis showed a significant reduction in the pain score of patients being treated with First Relief from start of the treatment to the end. This improvement persisted throughout the 90-day follow-up, suggesting that the treatment was a long-term improvement in neuropathic pain and not a short-term improvement.
The secondary outcome measures (VPT, Insomnia, ONLS and HAM) also showed similar improvements to the pain score, showing significant improvement in sleep and mood as the neuropathic pain decreased.
Reference:
FDA clears DyAnsys neurostimulation device first relief to treat diabetic neuropathic pain. News release. DyAnsys Inc. Accessed July 14, 2022. https://www.prnewswire.com/news-releases/fda-clears-dyansys-neurostimulation-device-first-relief-to-treat-diabetic-neuropathic-pain-301586766.html
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


