- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Nitroglycerin not to be given to patients with suspected stroke: LANCET
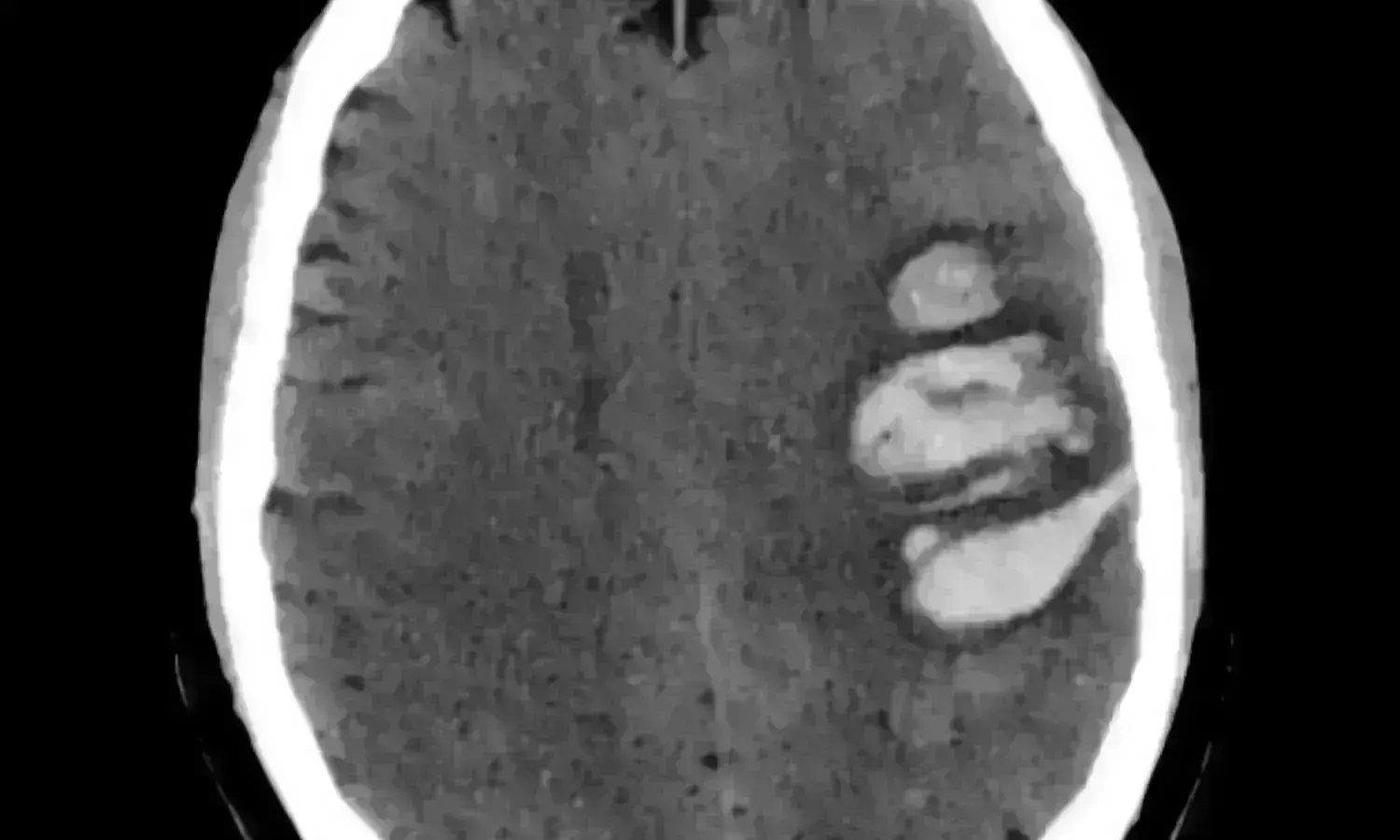
NETHERLANDS: According to a study published in The Lancet Neurology, giving glyceryl trinitrate to suspected acute stroke patients does not appear to change their functional prognosis, but it can be harmful to those who have intracerebral hemorrhages.
Glyceryl trinitrate, also known as nitroglycerin, may enhance functional outcomes in patients with acute ischemic stroke or intracerebral hemorrhage when administered exceptionally early. However, this conclusion was not supported by a more recent experiment.
The study's researchers sought to determine whether glyceryl trinitrate administered within three hours of symptom initiation benefits patients with suspected acute stroke.
In this phase 3, randomized, open-label, blinded endpoint trial, participants were randomized in a 1:1 ratio to receive either standard treatment alone (control group; n=155) or transdermal glyceryl trinitrate in addition to standard therapy (glyceryl trinitrate group; n=170). The main result was a functional outcome measured at 90 days using the modified Rankin Scale (mRS). Death in 7 days, death in less than 90 days, and significant adverse events were all safety outcomes. 1400 patients were the intended sample size.
380 individuals were assigned at random to one of two trial groups between April 4, 2018, and February 12, 2021. Of the 325 who gave informed consent or passed away before approval could be obtained, 170 were allocated to the glyceryl trinitrate group and 155 to the control group. With enrollment halted due to safety concerns in patients with intracerebral hemorrhage, the MR ASAP study was unexpectedly discontinued on June 24, 2021, at the recommendation of the data and safety monitoring board.
Conclusive findings of the study:
- A total of 201 patients (62%) experienced an ischemic stroke, 34 (10%) had a transient ischemic attack, 56 (17%) suffered an intracerebral hemorrhage, and 34 (10%) had a symptom that mimicked an ischemia stroke.
- At 90 days, the glyceryl trinitrate and control groups had median mRS scores of 2. The two trial groups did not differ regarding major adverse outcomes or death within 90 days.
- Compared to 2 (10%) of 21 patients in the control group, 12 (34%) of the 35 patients with intracerebral hemorrhage assigned to the glyceryl trinitrate group passed away within seven days.
"Glyceryl trinitrate should be avoided in this situation because there is a risk of potential early damage in individuals with intracerebral hemorrhage," added the authors.
In conclusion, there was no evidence of benefit for patients with suspected acute stroke when transdermal glyceryl trinitrate was initiated within three hours of the beginning of symptoms in the preclinical phase.
REFERENCE
van den Berg SA, Uniken Venema SM, Reinink H, Hofmeijer J, Schonewille WJ, Miedema I, et al. Prehospital transdermal glyceryl trinitrate in patients with presumed acute stroke (MR ASAP): an ambulance-based, multicentre, randomized, open-label, blinded endpoint, phase 3 trial. Lancet Neurol 2022:S1474442222003337. https://doi.org/10.1016/S1474-4422(22)00333-7.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


