- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Wearable,non-invasive, magnetic brain stimulation device could safely improve motor function after stroke
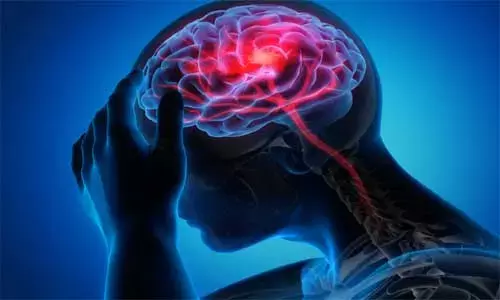
LOS ANGELES -Magnetic stimulation of the brain was previously investigated to promote recovery of motor function after stroke. The stimulation may change neural activity and induce reorganization of circuits in the brain.
Researchers have developed a wearable,non-invasive, magnetic brain stimulation device that could improve motor function in stroke patients. The research was presented at the American Stroke Association's International Stroke Conference 2020.
In an initial, randomized, double-blind, sham-controlled clinical trial of 30 chronic ischemic stroke survivors, a new wearable, multifocal, transcranial, rotating, permanent magnet stimulator, or TRPMS, produced significant increases in physiological brain activity in areas near the injured brain, as measured by functional MRI.
"The robustness of the increase in physiological brain activity was surprising. With only 30 subjects, a statistically significant change was seen in brain activity," said lead study author David Chiu, M.D., director of the Eddy Scurlock Stroke Center at Houston Methodist Hospital in Texas. "If confirmed in a larger multicenter trial, the results would have enormous implications. This technology would be the first proven treatment for recovery of motor function after chronic ischemic stroke."
Stroke survivors who had weakness on one side of their body at least three months post-stroke were enrolled in a preliminary study to evaluate safety and efficacy of the device. Half of the patients were treated with brain stimulation administered in twenty 40-minute sessions over four weeks. The rest had sham, or mock, treatment. Researchers analyzed physiologic brain activity before, immediately after and one month after treatment.
They found that treatment was well tolerated, and there were no device-related complications. Active treatment produced significantly greater increases in brain activity: nearly 9 times higher than the sham treatment.
Although the study could not prove that the transcranial stimulator improved motor function, numerical improvements were demonstrated in five of six clinical scales of motor function, as measured by a functional MRI test. The scales measured gait velocity, grip strength, pinch strength, and other motor functions of the arm. The treatment effects persisted over a three-month follow-up.
The researchers believe the study results are a signal of possible improved clinical motor function after magnetic brain stimulation for patients after stroke, which will need to be confirmed in a larger, multicenter trial.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


