- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Woven Endobridge device- New device for treatment of brain aneurysms
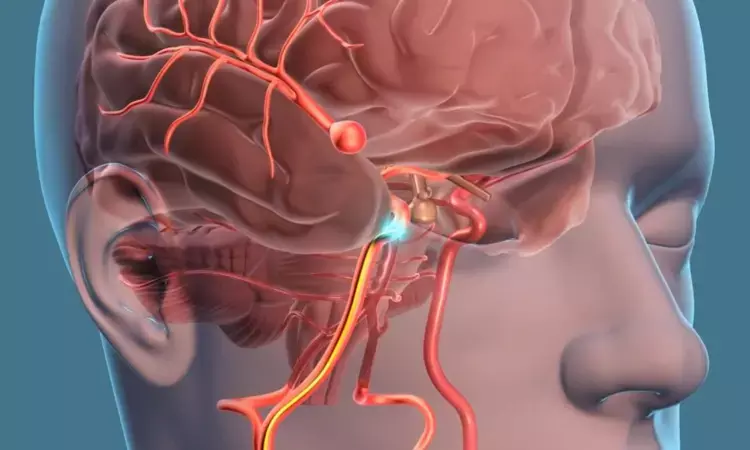
BOSTON - A recent international study led by researchers at Massachusetts General Hospital (MGH) and other institutions offers real-world results on the use of a novel device for treating brain aneurysms, or balloon-like bulges on weakened blood vessels that can rupture to cause life-threatening bleeding.
The study, which is published in Stroke, involved the use of the Woven Endobridge (WEB) device. This device consists of a tiny mesh plug that can be inserted through a vessel and placed into an aneurysm to block, or occlude, blood from going into the bulging region—thereby stabilizing the vessel and preventing the aneurysm from rupturing. The device can also be used to stabilize previously ruptured aneurysms.
"The WEB has recently become available in the United States, and elsewhere across the globe the device has been available for a longer time—but no large-scale study of its efficacy had yet been performed," says lead author Adam A. Dmytriw, MD, MPH, MSc, an interventional neuroradiology and endovascular neurosurgery fellow at MGH. "Thus, we founded the WorldWideWEB Consortium uniting the experience of 22 tertiary/quaternary institutions across North America, Europe, South America, and Australia."
The team found that among 671 patients with 683 brain aneurysms (26.2% previously ruptured) who were treated with the device and were followed for a median of 11 months, adequate occlusion occurred in 85.7% of aneurysms, and complete occlusion in 57.8%. Retreatment was required in 7.8% of aneurysms. Blood clot–related complications developed in 7.5% of procedures, of which only 4.0% were symptomatic and 2.0% were permanent. Bleeding complications occurred in 3.0% of procedures. No patients experienced aneurysm re-rupture after treatment.
"Patients who are not suitable for open aneurysm surgery or who have recently had a life-threatening rupture and would be at high risk for additional bleeding if treated with conventional endovascular techniques due to the need for blood thinners, now have a viable treatment option," says Dmytriw. "We hope that our results will help guide interventionalists in the appropriate use of the WEB so that patients with challenging brain aneurysms will have a safe option for care."
Addition medical institutions continue to join the consortium, which plans to conduct additional studies to assess the use of the WEB for diverse types of aneurysms and to examine potential challenges to its use.
https://www.ahajournals.org/doi/10.1161/STROKEAHA.121.037609
Hina Zahid Joined Medical Dialogue in 2017 with a passion to work as a Reporter. She coordinates with various national and international journals and association and covers all the stories related to Medical guidelines, Medical Journals, rare medical surgeries as well as all the updates in the medical field. Email: editorial@medicaldialogues.in. Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


